A trial of lenvatinib and pembrolizumab for head and neck cancer (LEAP-009)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is comparing lenvatinib and pembrolizumab with  for cancer of the head and neck. It is also looking at lenvatinib on its own.
for cancer of the head and neck. It is also looking at lenvatinib on its own.
The trial is for people whose cancer has come back or spread after previous treatment.
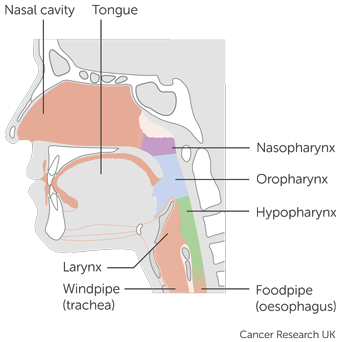
It includes people who have one of the following types of head and neck cancer:
- mouth and oropharyngeal cancer
- cancer of the area that surrounds the voice box (
hypopharynx  )
) - cancer of the voice box (laryngeal cancer)
More about this trial
Researchers are looking for ways to improve treatment for people who have cancer of the head and neck. In this trial they are looking at pembrolizumab and lenvatinib.
Pembrolizumab is an  . It helps the
. It helps the  to find and kill cancer cells.
to find and kill cancer cells.
Lenvatinib is a  . It stops signals that cancer cells use to divide and grow. It also stops cancer cells from forming new blood vessels, which they need to keep growing.
. It stops signals that cancer cells use to divide and grow. It also stops cancer cells from forming new blood vessels, which they need to keep growing.
Pembrolizumab is already used in the UK for some types of head and neck cancer but lenvatinib isn’t. Lenvatinib is a treatment for some other types of cancer.
In this trial, some people have lenvatinib and pembrolizumab. Some have standard treatment and some have only lenvatinib.
The standard treatments in this trial are:
- docetaxel chemotherapy
- paclitaxel chemotherapy
- capecitabine chemotherapy
- cetuximab (a
targeted drug  )
)
The main aims of the trial are to find out:
- how safe it is to have lenvatinib and pembrolizumab
- how well lenvatinib and pembrolizumab work compared to standard treatment
- how well lenvatinib works on its own
- more about the side effects of lenvatinib and pembrolizumab
- more about how treatment affects
quality of life 
Who can enter
The following bullet points are a main summary of the entry conditions for this trial. Talk to your doctor or the trial team if you are unsure about any of these. They will be able to advise you.
Who can take part
You may be able to join this trial if you have one of the following types of head and neck cancer:
- mouth and oropharyngeal cancer
- cancer of the area that surrounds the voice box (
hypopharynx  )
) - cancer of the voice box (laryngeal cancer)
And all of the following must also apply. You:
- have cancer that has come back after treatment and you can’t have surgery or radiotherapy with the aim to cure it or your cancer has spread elsewhere in the body
- have cancer that got worse while you were having a
platinum drug  such as carboplatin or cisplatin
such as carboplatin or cisplatin - have cancer that got worse after having certain
checkpoint inhibitor drugs  or certain
or certain monoclonal antibodies  . Your doctor will know this.
. Your doctor will know this. - have cancer that your doctor can see and measure on scans
- have cancer that has been tested for the
human papilloma virus (HPV  ) if you have cancer of the back of the mouth (oropharyngeal cancer)
) if you have cancer of the back of the mouth (oropharyngeal cancer) - have cancer that is
squamous cell cancer 
- have a sample of tissue (
biopsy  ) available from when you were diagnosed or you are willing to give a new sample
) available from when you were diagnosed or you are willing to give a new sample - can swallow tablets or have liquid treatment through a feeding tube if you have one
- are fit and active but you might not be able to do heavy physical work (performance status of 0 or 1)
- are willing to use reliable contraception during treatment and for a period afterwards if there is any chance you or your partner could become pregnant
- have satisfactory blood test results
- are at least 18 years old
Who can’t take part
Cancer related
You cannot join this trial if any of these apply. You:
- have nasopharyngeal cancer, salivary gland cancer or your doctors don’t know where your cancer started (cancer of unknown primary)
- have had repeated radiotherapy to the head and neck for your cancer including nearby
lymph nodes 
- have cancer that is getting worse quickly. For example your cancer is bleeding, has bled in the last 6 months or is causing pain
- have cancer that is growing into a major blood vessel or is very near to one
- have cancer that has broken through the skin and caused ulcers or wounds on your skin
- have had lenvatinib in the past
- have had chemotherapy, certain
targeted drugs  or radiotherapy within 2 weeks of starting treatment and you have certain ongoing side effects. This is apart from hair loss or mild to moderate numbness and tingling in the hand and feet (
or radiotherapy within 2 weeks of starting treatment and you have certain ongoing side effects. This is apart from hair loss or mild to moderate numbness and tingling in the hand and feet (peripheral neuropathy  )
) - have had more than 4 treatments for your cancer to the whole body in the past (
systemic treatment  )
) - have cancer that has spread to the brain, or spinal cord unless it isn’t causing symptoms, has been treated and is stable
- have another cancer that is getting worse or you have had treatment in the past 3 years. This is apart from
non melanoma skin cancer  ,
, early  bladder cancer, carcinoma insitu (
bladder cancer, carcinoma insitu (CIS  ) of the breast or cervix that has been successfully treated or early prostate cancer that is being monitored but you aren’t having treatment.
) of the breast or cervix that has been successfully treated or early prostate cancer that is being monitored but you aren’t having treatment. - have taken part in another trial looking at an experimental drug or device within 4 weeks of having treatment in this trial
Medical conditions
You cannot join this trial if any of these apply. You:
- have or had scarring or inflammation of the lungs (
pneumonitis  ) that needed steroid treatment
) that needed steroid treatment - have a problem with your
immune system  or have had treatment that damps down the immune system. This includes steroids within one week of starting trial treatment unless it is a low dose.
or have had treatment that damps down the immune system. This includes steroids within one week of starting trial treatment unless it is a low dose. - have an active
autoimmune condition  that needed treatment in the past 2 years apart from certain ones. Your doctor will know this.
that needed treatment in the past 2 years apart from certain ones. Your doctor will know this. - have had an
organ transplant 
- have had a bone marrow or stem cell transplant from another person (
allogeneic transplant  )
) - have HIV, an active hepatitis B or hepatitis C infection or any severe infection that needs treatment
- have high blood pressure that isn’t well controlled with medication
- have a severe
fistula 
- you have a problem with your
digestive system  that means you can’t absorb medication taken by mouth
that means you can’t absorb medication taken by mouth - have had major surgery within 3 weeks of having treatment in the trial
- have had a major
heart problem  such as a heart attack in the last year or another significant heart problem that needs treatment. The trial team check if you have a heart condition before you join the trial.
such as a heart attack in the last year or another significant heart problem that needs treatment. The trial team check if you have a heart condition before you join the trial. - have a heart scan that shows your heart isn’t working well
- have had a stroke in the last year
- have protein in your urine. Your doctor will check this.
- have any other medical condition or mental health problem that might affect you taking part in the trial
Other
You cannot join this trial if any of these apply. You:
- are allergic to pembrolizumab, lenvatinib, any of the standard treatments in this trial or anything they contain
- have had a live
vaccine  within 30 days of the start of treatment. Please note that the COVID-19 vaccine is allowed as it isn’t a live vaccine.
within 30 days of the start of treatment. Please note that the COVID-19 vaccine is allowed as it isn’t a live vaccine. - are pregnant or breastfeeding
Trial design
This phase 2 trial is taking place worldwide. The team need to find 400 people to take part including 18 from the UK.
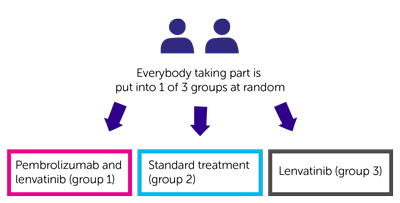
It is a randomised trial. You are put into a group by a computer. Neither you nor your doctor will be able to decide which group you are in. There are 3 treatment groups.
You have 1 of the following:
- pembrolizumab and lenvatinib (group 1)
- standard treatment (group 2)
- lenvatinib (group 3)
You have pembrolizumab as a drip into a vein. You have it once every 3 weeks. You have treatment for up to 2 years. After the 2 years you might be able to have another year of pembrolizumab. Your doctor will talk to you about this.
Lenvatinib is a capsule. You take it once a day every day. You have treatment for as long as it is working and the side effects aren’t too bad.
The standard treatments include 1 of the following:
- docetaxel chemotherapy
- paclitaxel chemotherapy
- capecitabine chemotherapy
- a targeted drug called cetuximab
Which standard treatment you have depends on what your doctor thinks is best for you.
You have docetaxel, paclitaxel or cetuximab as a drip into a vein. You have paclitaxel or cetuximab once a week for 3 weeks. You have docetaxel once every 3 weeks. Each 3 week period is a  .
.
Capecitabine is a tablet. You take it twice a day for 2 weeks. You then have a week of not taking it. This is a cycle of treatment.
Blood and tissue samples
The researchers ask you to give some extra blood samples. Where possible, you have these at the same time as your routine blood tests. They also ask to use a sample of tissue you gave for research.
They plan to use the samples to:
- see how well the treatment is working
- look at
genes  and
and DNA  to understand more about head and neck cancer
to understand more about head and neck cancer - look for substances called
biomarkers  to help work out why treatment might work for some people and not for others
to help work out why treatment might work for some people and not for others
Quality of life
The trial team ask you to fill out a questionnaire:
- before you start treatment
- at set times during treatment
The questionnaire asks about side effects and how you’ve been feeling. This is called a quality of life study
Hospital visits
You see the doctor and have tests before you can take part. These include:
- blood tests
- a
physical examination 
- heart trace (
ECG  )
) - heart scan (
echocardiogram  ) or
) or MUGA scan 
- bone scan
- CT scan or an MRI scan
You see the trial team for regular check ups and blood tests during treatment.
You have a CT scan or an MRI scan:
- every 6 weeks for the first year and then
- every 9 weeks after that
When you finish treatment you see the doctor 1 month later for a check up.
Follow up
The team follow you up every:
- 6 weeks for the first year and then
- 9 weeks after that
The trial team may also contact you every 3 months. You might see them at a routine hospital appointment or they may call you to see how you are getting on.
Side effects
The trial team monitor you during treatment and afterwards. Contact your advice line or tell your doctor or nurse if any side effects are bad or not getting better.
|
Pembrolizumab can affect the immune system. It may cause inflammation in different parts of the body. This can cause serious side effects. They could happen during treatment, or some months after treatment has finished. Rarely, these side effects could be life threatening. If you have any of these side effects tell your doctor or nurse as soon as possible. You should tell them that you are on or have been on an immunotherapy. |
The most common side effects of pembrolizumab are:
- skin rash, itchy skin or loss of skin colour
- diarrhoea
- cough
- joint, back or stomach pain
- high temperatures (fever)
- not enough
thyroid  hormone so you may feel tired, gain weight, feel cold or have constipation
hormone so you may feel tired, gain weight, feel cold or have constipation - low levels of salt in the blood that may cause tiredness, confusion, headaches, muscle cramps or feeling or being sick
The most common side effects of lenvatinib are:
- high or low blood pressure
- loss of appetite, weight loss or taste changes
- feeling or being sick
- constipation or diarrhoea
- tiredness or weakness (fatigue)
- dry, sore or inflamed mouth or throat
- high levels of protein in the urine
- hoarse voice
- headache
- sore or red palms of the hand and or soles of the feet (hand foot syndrome)
- joint, muscle, back, arm or leg pain
- a cough
- an increased risk of bruising or bleeding
- swelling of the legs
- not enough
thyroid  hormone causing tiredness, weakness, dry skin, hair loss and feeling cold
hormone causing tiredness, weakness, dry skin, hair loss and feeling cold - skin rash
- feeling dizzy
- problems with sleeping
- hair loss
- urine infections
- low levels of calcium and potassium in the blood which can cause problems with your heartbeat
| Please note, there are some other serious side effects of lenvatinib. In some cases these could be life threatening. You doctor will tell you more about these and what to watch out for. You should tell your trial doctor or go to the hospital straight away if you have any of them. |
These include:
- having a stroke or a mini stroke causing symptoms such as weakness or numbness on one side of the body
- blood clots in the legs causing a warm or swollen calf
- blood clots in the lungs causing shortness of breath
- heart problems such as a rapid heartbeat or a heart attack
- bleeding inside the body causing black or bloody poo
- a
fistula 
- low levels of fluid in the body (dehydration) and your kidneys not working well
- heart failure which may cause severe shortness of breath
- liver damage which may cause yellowing of the skin or eyes (jaundice), tiredness or sickness, loss of appetite, tummy pain or high temperature
- brain swelling that may cause confusion, drowsiness, poor concentration or loss of consciousness
The trial doctor will talk to you about all the possible side effects of treatment. You will have a chance to ask any questions you may have.
We have more information about:
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Petra Jankowska
Supported by
Merck, Sharp & Dohme
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040