Find a clinical trial
This diagram shows the process that researchers follow to set up and run a clinical trial. There is more information about each of the stages below.
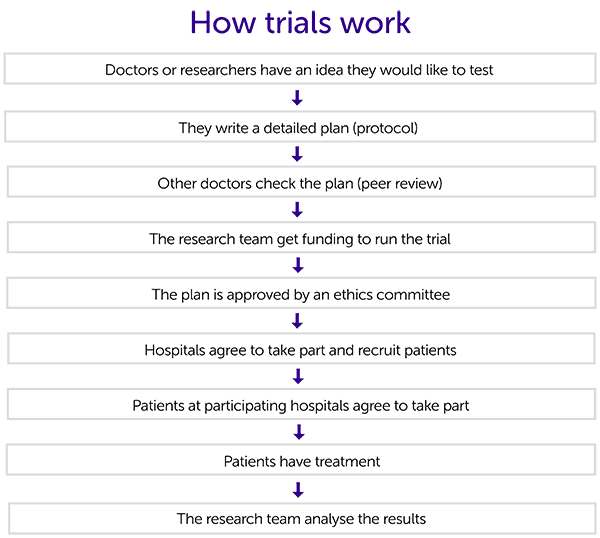
Having an idea
Researchers or doctors have an idea of something they would like to test. This could come from laboratory work, other trial results or experience with patients. The idea could involve:
a new treatment
a different combination of treatments
using an existing treatment for a different condition
testing a new way of giving an existing treatment
The team interested in doing the trial write a detailed plan (protocol) that everyone involved in the trial will use. It contains lots of information, including:
why they want to do the trial
who will be able to take part (the eligibility criteria)
how many people they need to take part
what the treatments are
what tests and appointments people will have before, during and after treatment
how and when they plan to analyse the results
Doctors, researchers and statistics experts who are not involved with the trial then check the protocol to make sure it is ok. They look at all aspects of the trial including:
how important the research question is
how easy it will be to find people to take part
whether they plan to analyse the results in the right way
if there are any possible difficulties the research team have not thought of
The research team will need money to run the trial. This may come from a charity, the government or a pharmaceutical company. We have information about how trials are funded.
All trials should have a sponsor. The sponsor is usually an organisation or it can be an individual. Some trials might have more than one sponsor and these are called co-sponsors. The sponsor is responsible for the overall management of the trial. This includes making sure that the trial team follow research regulations. And that the results are analysed and reported properly.
The protocol must be approved by an ethics committee. They must make sure it is in the best interest of the patients and will be possible to run. You can read more about how trials are approved.
Once the trial is approved, hospitals with the right expertise and equipment can sign. The research team will meet with the hospital staff to discuss the trial in more detail.
All clinical trials have entry conditions, known as . Once the trial is running, patients who fit the criteria can take part.
The research team must give clear information to anyone thinking about taking part. This includes what the trial involves and all the possible pros and cons.
Once they have read the information and asked any questions, patients sign a consent form to say they are happy to join the trial. We have information about what the trial team should tell you.
You can usually start treatment once you have signed the consent form. You may need to have some tests done before your treatment. The research team will go through your treatment plan with you.
Depending on the design of the trial, people may be put into one of the treatment groups at random. This makes sure the different trial groups are even and means the results are more reliable. You can read about what a randomised trial is.
Once everyone taking part has had treatment, the research team will look at and analyse the results. They will then draw some conclusions about the treatments in the trial. And they may make recommendations for future research.
Many research teams publish the results in a medical journal or present them at a conference. This is to share what they have learnt.
Sometimes the results show that the new treatment isn't better than the existing treatment. Even so, it adds to our knowledge and understanding of cancer and how to treat it. The results may also help decide what should be done in the next trial.
You can read more about clinical trial results and what the results mean.
If the results of the trial show that a new treatment is better than the existing treatment, the new treatment may be licensed. Doctors can then prescribe it. Licensing new treatments is quite a long and complicated process. It often depends on the results of more than one trial.
You can find out more about how drugs are licensed.
The (DMC) is a group of people who are not directly involved with the trial. They check on how things are going throughout the trial, and make sure everything is running safely. They can change parts of the trial, or even stop the trial, if they feel they need to.
You can learn more about how trials are monitored.
Last reviewed: 16 Jul 2025
Next review due: 16 Jul 2028

About Cancer generously supported by Dangoor Education since 2010. Learn more about Dangoor Education
Search our clinical trials database for all cancer trials and studies recruiting in the UK.
Connect with other people affected by cancer and share your experiences.
Questions about cancer? Call freephone 0808 800 40 40 from 9 to 5 - Monday to Friday. Alternatively, you can email us.