What are clinical trials
The video below shows how a randomised trial works. It is about 2 minutes long.
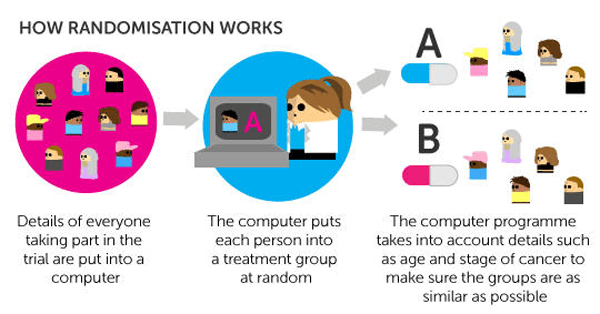
Randomised trials have at least 2 different treatment groups. The people taking part are put into one of the groups at random. This process is called 'randomisation' and is usually done by a computer. Most phase 3 trials and some phase 2 trials are randomised.
Often there is one group who have the standard treatment they would have if they weren’t in the trial. They are the control group. People in the other group (or groups) have a new treatment or procedure that is being tested. A randomised trial that has a control group is called a randomised controlled trial (RCT). Sometimes there is no standard treatment available for the control group. This could be because people have already had all the treatments currently available. In this situation, the people in the control group would have a dummy treatment. This is called a .
Randomised trials are used because they are the most reliable way to find out whether a treatment works. Randomisation helps to make sure that there is no that could distort the results. Of course, researchers are unlikely to be deliberately biased. But it is possible to be biased without realising it.
For example, doctors may avoid putting patients who are more unwell into a particular trial group without really meaning to. The people in this group then may not do as well as people in another group. This is because they were more unwell to begin with. The results would look as if the one treatment works better than the other, but really it doesn't.
The research team put information about the people taking part into the computer program. This might include the following:
your age
gender
the size of your cancer and how far it has spread (stage)
This makes sure that the groups are as similar as possible. Then the researchers know that if one group does better than the other, it’s because of the different treatment. And not because of general differences in the people taking part.
A placebo is a dummy treatment that has no active drug in it. They are used in some research trials. For example, one group of patients have the new treatment, and another group have the placebo. The placebo looks exactly like the actual treatment. If it is a pill or injection, it is the same shape, colour and size.
The results of both groups are then compared to find out how well the new treatment works.
Researchers only use a placebo if there is no standard treatment available. The patients in the control group wouldn't have any treatment if they weren't in the trial. So they are not missing out on treatment they would otherwise have had.
It isn't ethical to give a placebo to a group of people who really need treatment for cancer. The research ethics committee would not give permission for a trial designed in that way. We have information about how trials are approved.
A blind trial is a trial where the people taking part don't know which treatment they are getting. They could be one of the following:
the new treatment
the standard treatment
a placebo
This depends on the design of the trial. All patients have identical injections or tablets, so they can't tell which treatment they are having.
A double blind trial is a trial where neither the researchers nor the patients know what they are getting. The computer gives each patient a code number. And the code numbers are then allocated to the treatment groups. Your treatment arrives with your code number on it. Neither you nor your doctor knows whether it is the new treatment or not.
The list of patients and their code numbers are kept secret until the end of the trial. In an emergency, the researchers can find out which trial group a patient is in. This is to check that the emergency hasn’t been caused by the trial drug. But generally no one knows which treatment you had until the trial has finished.
Last reviewed: 17 Jul 2025
Next review due: 17 Jul 2028

About Cancer generously supported by Dangoor Education since 2010. Learn more about Dangoor Education
Search our clinical trials database for all cancer trials and studies recruiting in the UK.
Connect with other people affected by cancer and share your experiences.
Questions about cancer? Call freephone 0808 800 40 40 from 9 to 5 - Monday to Friday. Alternatively, you can email us.