A study to find out how long people with melanoma should have treatment with pembrolizumab and nivolumab (DANTE)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This study is for people with melanoma that has spread to other parts of the body (metastatic).
More about this trial
 ) to find and destroy the cancer. Pembrolizumab (Keytruda) and nivolumab (Opdivo) are 2 possible immunotherapy treatments.
) to find and destroy the cancer. Pembrolizumab (Keytruda) and nivolumab (Opdivo) are 2 possible immunotherapy treatments. - stop pembrolizumab or nivolumab after 1 year of treatment
- continue pembrolizumab or nivolumab for as long as doctors think it is still effective, the side effects aren’t too bad or for up to 2 years or for longer than 2 years
- reduce the number of side effects
- improve the
quality of life  of people with melanoma
of people with melanoma
Who can enter
- have melanoma that has spread to the nearby
lymph nodes  or to other parts of the body (stage 3 or stage 4) and cannot be completely removed with surgery
or to other parts of the body (stage 3 or stage 4) and cannot be completely removed with surgery - are going to have, or are having, pembrolizumab or nivolumab for the 1st time OR have started pembrolizumab or nivolumab on its own or nivolumab with ipilimumab less than a year ago
- are at least 18 years old
- you joined the 1st part of this study (registration)
- your cancer stayed the same or got better after 12 months of pembrolizumab or nivolumab
- you have had pembrolizumab or nivolumab for at least a year
- you are well enough to continue treatment with nivolumab or pembrolizumab
- you are well enough to be up and about for at least half the day (performance status 0, 1 or 2)
- you are willing to use reliable contraception during treatment and for 6 months afterwards if there is any possibility that you or your partner could become pregnant
- have had treatment that reached your whole body (systemic) for melanoma that has spread, unless it was given in addition to the main treatment such as surgery and you have finished it more than 6 months before the start of pembrolizumab or nivolumab
- have cancer spread in the brain and it is getting worse (progression) or you are taking high doses of corticosteroids such as prednisolone to help with the symptoms
- have had another cancer in the past 5 years apart from stage 1 or stage 2 non melanoma skin cancer, a
carcinoma in situ  that has been successfully treated or any other cancer that has been successfully treated and there has been no sign of it for more than a year
that has been successfully treated or any other cancer that has been successfully treated and there has been no sign of it for more than a year
- have active HIV
- have active hepatitis B or hepatitis C
- have an active infection that needs treatment that reaches your whole body (systemic)
- have a severe
auto immune disease  or inflammation of the lung tissue (
or inflammation of the lung tissue (pneumonitis  )
) - have any other severe condition that the trial team think could affect you taking part
- are pregnant or breastfeeding
Trial design
- registration
- the study itself (randomisation)
- are about to start pembrolizumab or nivolumab
- have been having pembrolizumab or nivolumab for up to 12 months
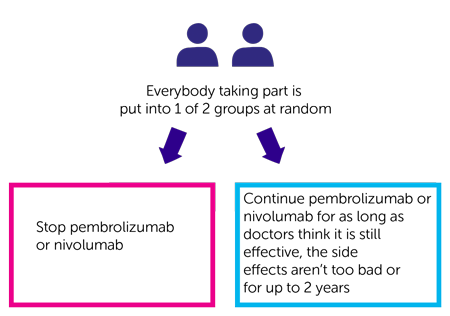
- stop pembrolizumab or nivolumab (experimental group)
- continue pembrolizumab or nivolumab for as long as doctors think it is still effective, the side effects aren’t too bad or for up to 2 years or for longer than 2 years (treatment group)
- before finding out which group they go to
- then every 3 months for up to 18 months
Hospital visits
- every 3 months for the first year
- then every 6 months for 3 years
Side effects
- skin rash, itchy skin and changes to your skin colour
- fatigue (tiredness)
- loss of appetite
- shortness of breath and cough
- pain in your joints and back
- high temperature (fever)
- swelling in the legs and feet
- low levels of salt in your body that can cause muscle cramps and confusion
- feeling or being sick
- diarrhoea or constipation
- a drop in the number of red blood cells causing breathlessness and looking pale
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Sarah Danson
Supported by
Sheffield Teaching Hospitals NHS Foundation Trust
The University of Sheffield
Leeds Institute of Clinical Trials Research
University of Leed
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040