A trial to find the best way of giving an increased dose of radiotherapy to treat non small cell lung cancer (ADSCaN)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is for people who have locally advanced non small cell lung cancer and are having chemotherapy followed by radiotherapy.
More about this trial
 or nearby structures in the chest, such as the windpipe.
or nearby structures in the chest, such as the windpipe.  radiotherapy is every day, Monday to Friday for 4 weeks.
radiotherapy is every day, Monday to Friday for 4 weeks. - CHART-ED
- IDEAL
- I-START
- Isotoxic IMRT
 using this way.
using this way.Who can enter
- have non small cell lung cancer that is stage 3
- have had 2 cycles of chemotherapy including cisplatin or carboplatin (platinum chemotherapy) and your cancer had responded or stayed the same
- have satisfactory breathing test (pulmonary function) results
- are up and about for more than half the day (performance status 0, 1 or 2)
- are at least 16 years old
- are able to have surgery to remove your cancer
- are able to have chemotherapy at the same time as radiotherapy (
chemoradiotherapy  )
) - have another cancer that your doctor or the trial team think could affect you taking part
- have a medical condition that isn’t controlled such as diabetes, high blood pressure, infection or heart disease
- have a condition such as scleroderma or systemic
lupus erythematosus  that affects connective tissue
that affects connective tissue - have a disease that affects the tissue and space around the air sacs of the lung (interstitial lung disease) that is causing symptoms
- have another medical or mental health condition that could affect you taking part
- are pregnant or breastfeeding
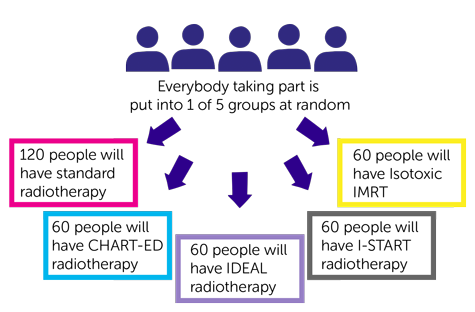
Trial design
- 120 people will have standard radiotherapy
- 60 people will have CHART-ED radiotherapy
- 60 people will have IDEAL radiotherapy
- 60 people will have I-START radiotherapy
- 60 people will have Isotoxic
IMRT 
- 3 treatments every day for 12 days including weekends
- 2 days (a weekend) of no treatment
- 2 treatments each day for 3 days
- 2 radiotherapy treatments one day a week
- 1 radiotherapy treatment on the other 4 days of the week
- 2 months
- 3 months
- 4 months
- 6 months
- then every 3 months to 18 months
- at 2 years
- then every year
Hospital visits
- a physical examination
- blood tests
- breathing tests
- PET scan (if needed)
- chest x-ray
- CT scan
- heart trace (
ECG  )
)
- 2 months
- 3 months
- 4 months
- 6 months
- then every 3 months to 18 months
- at 2 years
- then every year
Side effects
- inflammation of the food pipe (oesophagitis) that causes soreness when swallowing
- inflammation of the lung that causes coughing and shortness of breath
- a skin reaction where you are being treated causing redness and, or, soreness
- tiredness
- scarring or narrowing of the food pipe causing soreness and difficulty swallowing. Your doctor might be able to give you some medicine to help
- breathlessness caused by scarring of the lung tissue
- darkening or lightening of the skin where you had treatment
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Matthew Hatton
Supported by
Cancer Research UK
NHS Greater Glasgow and Clyde
National Radiotherapy Trials Quality Assurance (RTTQA) Group
Other information
This is Cancer Research UK trial number CRUK/14/040.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



