A trial using circulating tumour DNA to work out when to switch between targeted treatment and immunotherapy for melanoma (CAcTUS)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is looking at whether measuring  can help doctors work out the best time to switch treatment for people with melanoma.
can help doctors work out the best time to switch treatment for people with melanoma.
It is for people who:
- can’t have surgery to remove the melanoma or it has spread elsewhere in the body and
- have melanoma cells with a specific gene change in the BRAF gene called BRAF VE 600
More about this trial
You may have a targeted drug or an immunotherapy for melanoma that has spread and has the BRAF V600 gene change. The change to this gene causes it to make an overactive BRAF protein. This makes cells grow and divide too fast.
These treatments work well but they don’t work for everyone. Targeted treatment can stop working or only work for a short time. Using immunotherapy together with a targeted therapy can improve treatment. But this is only if the cancer hasn’t developed resistance.
The usual way to check how treatment is working is to have CT scans. You have these every few months. The trial team have now developed a new blood test. The blood test looks for pieces of the BRAF DNA in the melanoma cells. A lower level of BRAF DNA shows that treatment is working. A higher level shows that treatment isn’t working very well. The blood test can track changes in how treatment is working as they happen. And it can be done more often than a scan.
Researchers want to see if the test is good enough to help doctors work out when to switch between a targeted drug and immunotherapy. It is important that doctors switch treatments at the right time.
In this trial, the:
- targeted drugs you have are dabrafenib and trametinib
- immunotherapy drugs you have are nivolumab and ipilimumab
These are all usual treatments for melanoma that has spread.
The main aims of the trial are to:
- find out how easy it is to send the blood samples to the laboratory and get them analysed in time
- see how well the circulating tumour DNA (ctDNA) blood test works
Who can enter
The following bullet points are a summary of the entry conditions for this trial. Talk to your doctor or the trial team if you are unsure about any of these. They will be able to advise you.
Who can take part
You may be able to join this trial if all of the following apply. You:
- have melanoma that can’t be removed with surgery or it has spread elsewhere in the body (stage 3 to stage 4 melanoma)
- have a change (
mutation  ) in the BRAF V600 gene on your melanoma cells
) in the BRAF V600 gene on your melanoma cells - have at least one area of melanoma that your doctor can see and measure on a scan
- completed radiotherapy or
radiosurgery  at least 2 weeks before you have the first dose of trial treatment if you had this
at least 2 weeks before you have the first dose of trial treatment if you had this - have satisfactory blood test results
- are well enough to be up and about for at least half the day but you might not be able to work (performance status 0, 1 or 2)
- are willing to use reliable contraception during the trial and for a period after if there is any chance you or your partner could become pregnant
- are at least 18 years old
Who can’t take part
Cancer related
You cannot join this trial if any of these apply. You:
- have cancer that has spread to the brain or spinal cord or the tissues that surround the brain unless it isn’t causing symptoms, has been treated and is stable
- have had treatment for melanoma that can’t be removed by surgery or has spread elsewhere in the body. Treatments may have included immunotherapy, targeted treatment,
cancer vaccines  or an experimental treatment.
or an experimental treatment. - have already had treatment after surgery that included a combination of checkpoint inhibitors. For example you have had nivolumab and ipilimumab.
- have cancer that has spread to the heart or the blood vessels and arteries that surround it
- have had another cancer unless there have been no signs of it for 3 years or you had a
non melanoma skin cancer  that was successfully treated
that was successfully treated
Medical conditions
You cannot join this trial if any of these apply. You:
- have an inherited condition that causes
red blood cells  to break down. Your doctor will know this.
to break down. Your doctor will know this. - have an
autoimmune condition  apart from certain ones. Your doctor will know this.
apart from certain ones. Your doctor will know this. - have had treatment that damps down the immune system. This includes steroids within 2 weeks of starting trial treatment unless it was a low dose.
- have scarring on the lungs that is causing symptoms or may interfere with picking up any lung side effects that trial treatment may cause
- have had medication that stops you having fits (seizures) in the month before starting trial treatment
- have had a heart attack in the last 6 months, you have a fast heartbeat or you have another serious
heart problem  that needs treatment. You doctor checks if you have a heart condition before you can take part in the trial.
that needs treatment. You doctor checks if you have a heart condition before you can take part in the trial. - have high blood pressure and medication isn’t controlling it
- have HIV or an active hepatitis B or hepatitis C infection
- have a change in
electrolytes  that can’t be corrected with medication
that can’t be corrected with medication - have or have had a blockage in a vein in your eye or have a risk of developing this. Symptoms include problems with your eyesight. Your doctor will know this.
- have any other serious or uncontrolled medical condition or mental health problem that the trial team think would affect you taking part in the trial
Other
You cannot join this trial if any of these apply. You:
- are having treatment that can’t you have if you are in this trial. For example drugs to treat HIV or herbal medicine such as St John’s wort.
- are allergic to any of the treatments in the trial or anything they contain
- are pregnant or breastfeeding
Trial design
This is a pilot trial. The trial team need 40 people to take part.
Depending on the results, researchers may then carry out a larger trial to check how well the new blood test works in more people.
This is a randomised trial. A computer puts you into a treatment group. Neither you nor your doctor can decide which group you are in.
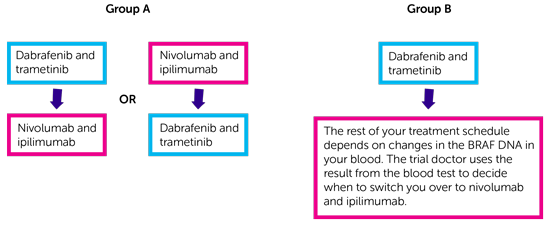
There are 2 treatment groups. You have 1 of the following.
- In group A you start by having dabrafenib and trametinib followed by nivolumab and ipilimumab. Or you start by having nivolumab and ipilimumab followed by dabrafenib and trametinib.
- In group B you start by having dabrafenib and trametinib. The rest of your treatment schedule depends on changes in the BRAF DNA in your blood. The trial doctor uses the result from the blood test to decide when to switch you over to nivolumab and ipilimumab.
Screening blood test
To begin with you have a blood test to check the level of BRAF DNA. It needs to be above a certain level for you to take part in the trial. You won’t be able to join the trial if the level isn’t high enough. Your doctor will talk to you about other treatment options.
How you have trial treatment
Dabrafenib are capsules and trametinib are tablets. You take dabrafenib twice a day, every day. You take trametinib once a day, every day.
You have nivolumab and ipilimumab as a drip into a vein. This takes about 90 minutes each time. You have the first 4 doses of both drugs every 3 weeks. You then stop ipilimumab and continue with only nivolumab. You have nivolumab once every 2 weeks. You continue to have it as long as it’s working and the side effects aren’t too bad.
Treatment in Group A
In group A you have:
- dabrafenib and trametinib followed by nivolumab and ipilimumab
or
- nivolumab and ipilimumab followed by dabrafenib and trametinib
Your doctor will discuss the treatment options with you. Together you decide which treatment you will have first. If your cancer gets worse or if you get too many side effects from the first treatment then you switch to the second one.
You have a short period of no treatment after stopping the first treatment. This is so that your body is free from drugs before starting the second treatment. You stop trial treatment if your melanoma gets worse on the second treatment. Your doctor will talk to you about other treatment options.
Treatment in group B
In group B your treatment schedule is based on changes in the BRAF DNA in your blood. You start by having dabrafenib and trametinib. You then have a blood test at:
- week 2
- week 4 and then
- every 4 weeks
Your doctor uses the blood test result to see if the BRAF DNA level is falling. This means the treatment is working.
When the level of BRAF DNA falls below a certain level, you switch treatment. There will be a short period of no treatment after stopping dabrafenib and trametinib. This is so that your body is free from drugs before starting nivolumab and ipilimumab.
You stop nivolumab and ipilimumab if the cancer comes back or you have bad side effects. Your doctor will switch you back to dabrafenib and trametinib. You have this as long as treatment is working and the side effects aren’t too bad.
You stop treatment if your cancer gets worse. Your doctor will talk to you about other treatment options.
Hospital visits
You see the doctor and have some tests before you can join the trial.
You have the BRAF DNA blood test first to check you have a certain level of BRAF. Once this is confirmed you have some further tests to check you are suitable to take part. These include:
You see the trial doctor for regular check ups and blood tests. You have a heart scan every 3 months while having dabrafenib and trametinib.
During treatment you have a CT or MRI scan every 2 months. This is to check how your treatment is working.
Follow up
When you finish treatment you have a check up every 3 months until a year after the last person who joined the trial started their treatment. After that you see the team every 6 months to see how you are or they might call you.
Side effects
The trial team monitor you during treatment and afterwards. Contact your advice line or tell your doctor or nurse if any side effects are bad or not getting better.
| Nivolumab and ipilimumab can affect the immune system. They may cause inflammation in different parts of the body. This can cause serious side effects. They could happen during treatment, or some months after treatment has finished. Rarely, these side effects could be life threatening. If you have any of these side effects tell your doctor or nurse as soon as possible. You should tell them that you are on or have been on an immunotherapy. |
The most common side effects of nivolumab and ipilimumab include:
- high temperatures (fever)
- swelling or inflammation
- skin problems such as a skin rash, blisters and peeling skin
- weight gain
- mood changes
- hair loss
- muscle and joint pain
- diarrhoea or constipation
- feeling or being sick
- tummy (abdominal) pain
- changes to how the liver and kidneys work
The most common side effects of dabrafenib and trametinib include:
- high temperatures (fever)
- skin problems such as soreness and redness of palms of hands and soles of feet and skin rash
- feeling or being sick
- diarrhoea or constipation
- fatigue (tiredness)
- muscle and joint pain
- headache
- cough
The trial doctor will talk to you about all the possible side effects of treatment. We have more information about:
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Paul Lorigan
Supported by
Bristol-Myers Squibb
The Christie NHS Foundation Trust
University of Manchester
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040