Head and neck cancers, Laryngeal cancer, Mouth and oropharyngeal cancer
Results
Phase 1
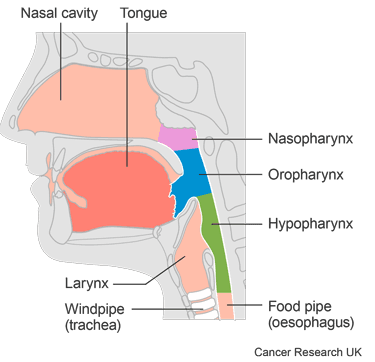
This trial looked at a drug called adavosertib (AZD1775) before surgery and after surgery for head and neck cancer. It was for people who had one of the following head and neck cancers:
cancer where the food pipe surrounds the voice box (hypopharynx)
The trial was open for people to join between 2017 and 2019. The team published the results in 2024.
Recruitment start: 30 October 2017
Recruitment end: 15 July 2019
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Professor Hisham Mehanna
AstraZeneca
Cancer Research UK
University of Birmingham
Last reviewed: 18 Mar 2024
CRUK internal database number: 14313