A trial looking at whether changing the dose of radiotherapy improves swallowing for people with head and neck cancer (DARS)
Cancer type:
Status:
Phase:
This trial looked at different doses of radiotherapy as part of chemoradiotherapy to treat throat (pharyngeal) cancer.
The trial was supported by Cancer Research UK. It was open for people to join between 2016 and 2018. The team published the results in 2023.
More about this trial
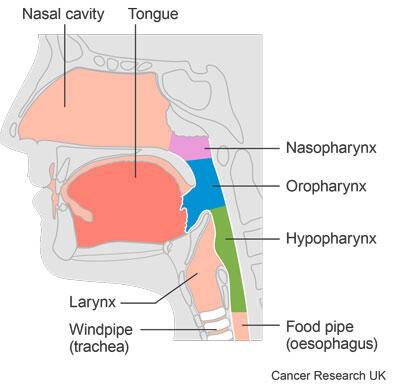
Doctors often use radiotherapy to treat head and neck cancers. This trial was for people with cancer of the oropharynx and hypopharynx. These are both part of the throat.
When this trial was done, intensity modulated radiotherapy (IMRT) was the standard treatment for head and neck cancer. This is a way of aiming the radiotherapy at the cancer but not the heathy tissue around it, to reduce side effects. Some people still have side effects, including difficulty swallowing (dysphagia).
Doctors wanted to find a way to reduce the side effects further. They hoped that changing the way they give IMRT to avoid the muscles that control swallowing would help. This is called dysphagia optimised IMRT (DO-IMRT).
Everyone in this trial had intensity modulated radiotherapy (IMRT):
- half had standard IMRT
- half had dysphagia optimised IMRT
The main aim of the trial was to find out if DO-IMRT causes fewer swallowing problems than standard IMRT.
Summary of results
This trial showed that dysphagia optimised IMRT (DO-IMRT) did cause fewer swallowing problems than standard IMRT.
Results
A total of 112 people were put into one of 2 groups at random. They all had head and neck cancer and were due to have radiotherapy.
There were:
- 56 people in the standard IMRT group
- 56 people in the DO-IMRT group
The trial team looked at whether people’s swallowing changed, and how this affected their  . They used a tool called the MD Anderson Dysphagia Inventory (MDADI).
. They used a tool called the MD Anderson Dysphagia Inventory (MDADI).
This questionnaire asks people how much their swallowing difficulties impact things such as their:
- day to day life
- social life
- self esteem
- food intake
- weight
- quality of life
It gives a score between 20 (low quality of life) and 100 (high quality of life).
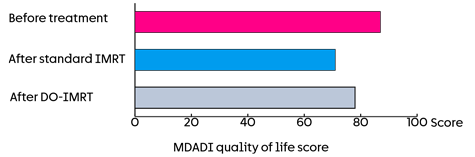
The results of this trial showed that the MDADI scores were about 87 for people in both groups before treatment.
A year after treatment they were:
- 71 for those who had standard IMRT
- 78 for those who had DO-IMRT
This means that changes in swallowing affected the quality of life less for those who had DO-IMRT.
Side effects
Everyone taking part had at least one side effect. Many were mild or didn’t last long. Some people in each group had more moderate or severe side effects.
These side effects included:
- hearing loss
- sore mouth or throat
- dry mouth
- weight loss
- difficulty swallowing
A few more people who had standard IMRT had these side effects. Overall, the number of people who had side effects was similar in the two groups.
Conclusion
The trial team concluded that DO-IMRT didn’t affect swallowing as much as standard IMRT. They suggest it could become the standard treatment for people with throat (pharyngeal) cancer.
More detailed information
There is more information about this research in the reference below.
Please note, the information we link to here is not in plain English. It has been written for healthcare professionals and researchers.
Dysphagia-optimised intensity-modulated radiotherapy versus standard intensity-modulated radiotherapy in patients with head and neck cancer (DARS): a phase 3, multicentre, randomised, controlled trial.
C Nutting and others
The Lancet Oncology, 2023. Volume 24, issue 8, pages 868 – 880.
Where this information comes from
We have based this summary on the information in the article above. This has been reviewed by independent specialists ( ) and published in a medical journal. We have not analysed the data ourselves. As far as we are aware, the link we list above is active and the article is free and available to view.
) and published in a medical journal. We have not analysed the data ourselves. As far as we are aware, the link we list above is active and the article is free and available to view.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Christopher Nutting
Supported by
Cancer Research UK
Institute of Cancer Research (ICR)
NIHR Clinical Research Network: Cancer
The Royal Marsden NHS Foundation Trust
Cancer Trials Ireland
Other information
This is Cancer Research UK trial number CRUK/14/014.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040