A trial looking at ruxolitinib for polycythaemia vera (The RESPONSE trial)
Cancer type:
Status:
Phase:
This trial looked at ruxolitinib (INC424) for people with polycythaemia vera who didn’t respond to standard treatment.
More about this trial
Polycythaemia vera is a myeloproliferative disorder. This is a condition where the  produces too many blood cells. In polycythaemia this is too many red blood cells.
produces too many blood cells. In polycythaemia this is too many red blood cells.
An  called JAK2 signals the bone marrow to produce more blood cells when needed. But in most people who have polycythaemia vera the JAK2 enzyme is faulty. So their bone marrow produces too many red blood cells.
called JAK2 signals the bone marrow to produce more blood cells when needed. But in most people who have polycythaemia vera the JAK2 enzyme is faulty. So their bone marrow produces too many red blood cells.
Doctors treat polycythaemia vera with drugs such as hydroxycarbamide (hydroxyurea). This can control the number of blood cells. But sometimes hydroxycarbamide stops working. Or people can’t carry on taking it because of unacceptable side effects.
Researchers looked for new treatments to help these people.
In this trial they looked at ruxolitinib. It blocks the action of the JAK2 enzyme and might reduce the number of red blood cells. The aims of the study were to
- find if ruxolitinib helped people with polycythaemia vera
- learn more about the side effects
Summary of results
The trial team found that ruxolitinib was better than standard treatment for people with polycythaemia vera (PV).
This phase 3 trial recruited 222 people. It was a randomised trial.
- 110 people had ruxolitinib
- 112 people had standard treatment
The team looked at 2 signs of PV to see what the response to treatment was
- size of the
spleen 
- a blood test looking at the level of haematocrit
People who have PV have an enlarged spleen. They also have a high level of haematocrit.
After 32 weeks of treatment the team wanted to see how many people’s spleen had reduced in size by at least 35%. And whose haematocrit level was under control.
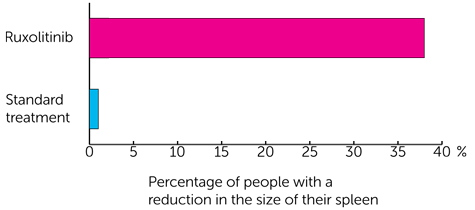
For reduction in spleen size it was
- 38 out of every 100 people (38%) had ruxolitinib
- 1 out of every 100 people (1%) had standard treatment
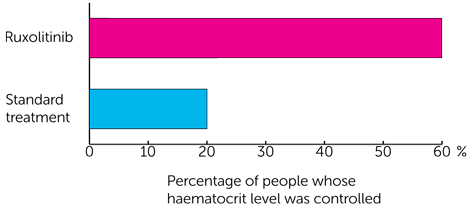
For control of haematocrit level it was
- 60 out of every 100 people (60%) had ruxolitinib
- 20 out of every 100 people (20%) had standard treatment
The most common side effects of ruxolitinib were
- a drop in blood cells
- headache
- diarrhoea
- tiredness
- itching
- dizziness
- muscle spasms
- shortness of breath
- tummy (abdominal) pain
The trial team concluded that ruxolitinib was better than standard treatment for PV at
- controlling the haematocrit level
- reducing the size of the spleen
- improving the symptoms of PV
We have based this summary on information from the research team. The information they sent us has been reviewed by independent specialists ( ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Claire Harrison
Supported by
Incyte Corporation
NIHR Clinical Research Network: Cancer
Novartis
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



