A trial looking at ruxolitinib before a stem cell transplant for myelofibrosis (MPD-RC114)
Cancer type:
Status:
Phase:
This trial looked at ruxolitinib before a stem cell transplant to improve treatment for people with myelofibrosis.
Myelofibrosis is a rare blood disorder. It is a condition that causes scarring of the  . A small number of people with myelofibrosis go on to develop
. A small number of people with myelofibrosis go on to develop  .
.
This trial was open for people to join between 2015 and 2017. These results were published in 2018.
More about this trial
 The aim is to try to cure it. Doctors are always looking for ways to improve treatment. In this trial, they looked at a drug called ruxolitinib.
The aim is to try to cure it. Doctors are always looking for ways to improve treatment. In this trial, they looked at a drug called ruxolitinib.- find out if having ruxolitinib before a stem cell transplant improved treatment
- learn more about the side effects
Summary of results
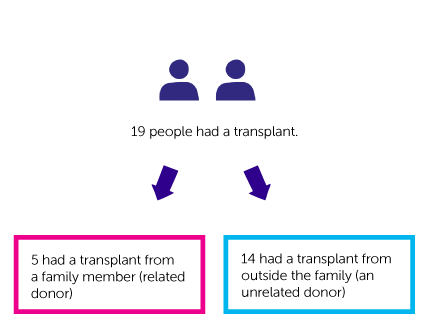
- 5 had a transplant from a family member (related donor)
- 14 had a transplant from outside the family (an unrelated donor)
The study closed earlier than planned. This was because:
- it was difficult to find enough people to join
- treatment wasn’t working as well as researchers had hoped
- a drop in the number of red blood cells (anaemia)
- an increased risk of bleeding
 disease. This is a side effect of having a transplant.
disease. This is a side effect of having a transplant. - within 100 days of having a transplant, 12 people had developed short term graft versus host disease
- after 100 days, 11 had long term graft versus host disease
 ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Adam Mead
Supported by
Bloodwise
NIHR Clinical Research Network: Cancer
Oxford University Hospitals NHS Trust
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040