A trial looking at targeted cancer drugs or chemotherapy before surgery for locally advanced bowel cancer (FOXTROT 4)
Cancer type:
Status:
This trial is looking at  before surgery for bowel cancer to improve treatment.
before surgery for bowel cancer to improve treatment.
It is for people who have all the following:
- bowel cancer that has spread into the surrounding tissues (
locally advanced cancer  )
) - a BRAF
gene  change (mutation) called BRAFV600E
change (mutation) called BRAFV600E - a plan to have surgery to remove their cancer
More about this trial
The main treatment for locally advanced bowel cancer with a BRAF gene change is a combination of  and surgery.
and surgery.
Researchers think that having two targeted cancer drugs before surgery might work better than chemotherapy before surgery. But they aren’t sure. They are doing this trial to try and find out.
Targeted cancer drugs work by targeting those differences that help a cancer cell to survive and grow. The trial team are looking at the targeted cancer drugs encorafenib and cetuximab. This combination is already a usual treatment for some people whose bowel cancer has spread to other parts of the body.
Encorafenib is a type of cancer growth blocker. It works by targeting certain proteins that help cancer cells grow. By blocking these proteins, it stops or slows down the growth of cancer cells.
Cetuximab is a type of  . It seeks out cancer cells by targeting particular proteins on their cell surface.
. It seeks out cancer cells by targeting particular proteins on their cell surface.
In this trial, some people have 2 chemotherapy drugs before surgery. These are oxaliplatin and a type of fluoropyrimidine chemotherapy (for example capecitabine or fluorouracil). And some people have encorafenib and cetuximab before surgery.
The main aims of the trial are to:
- see if the targeted cancer drugs can be given safely before surgery
- find out if targeted cancer drugs work better than chemotherapy before surgery
- learn more about the side effects
- see how treatment affects quality of life
Who can enter
The following bullet points are a summary of the entry conditions for this trial. Talk to your doctor or the trial team if you are unsure about any of these. They will be able to advise you.
Who can take part
You may be able to join this trial if all of the following apply.
- You have
adenocarcinoma  of the large bowel (colon) or upper back passage (rectum). Or you have bowel cancer cells that look very abnormal under the microscope and have grown into surrounding tissues.
of the large bowel (colon) or upper back passage (rectum). Or you have bowel cancer cells that look very abnormal under the microscope and have grown into surrounding tissues. - You have cancer that has grown into the outer lining of the bowel wall or further. It may or may not have spread into lymph nodes, but it hasn’t spread to other parts of the body. (This is stage T3-4, N0-2, M0 bowel cancer).
- You have a sample of tissue (
biopsy  ) available for the trial team to do some tests on.
) available for the trial team to do some tests on. - Your doctors are aiming to get rid of all of your cancer with treatment.
- You are suitable for surgery with
chemotherapy  around the time of surgery.
around the time of surgery. - You have not had treatment for bowel cancer before.
- You have a BRAF
gene  change (mutation) called BRAFV600E. Your doctor can tell you more.
change (mutation) called BRAFV600E. Your doctor can tell you more. - You have satisfactory blood test results.
- You are willing to use reliable contraception during the trial and for 6 months after if there is any chance you or your partner could become pregnant.
- You are at least 18 years old.
Who can’t take part
Cancer related
You cannot join this trial if any of these apply. You:
- are going to have
radiotherapy 
- might have cancer that has spread to other parts of the body or into the
lymph nodes  in the
in the peritoneum 
- have, or are developing, a blockage in your bowel
- have had another cancer in the last 5 years apart from
non melanoma skin cancer  , very early stage cancer (
, very early stage cancer (carcinoma in situ  ) or an early stage cancer that is unlikely to come back
) or an early stage cancer that is unlikely to come back - have microsatellite instability high (MSI-H) or mismatch repair deficiency (dMMR) bowel cancer. Your doctor will know this.
- have had treatment with targeted cancer drugs called RAF inhibitors or EGFR inhibitors before. Your doctor can tell you more.
Medical conditions
You cannot join this trial if any of these apply. You:
- have tingling or numbness in your hands or feet and this causes significant problems with activities of daily living
- have a
DPD deficiency 
- have a severe inflammatory bowel disease
Other
You cannot join this trial if any of these apply. You:
- have taken certain anti-viral medications or St John’s Wort recently. Your doctor will know more.
- are allergic to the chemotherapy drug oxaliplatin or to a group of chemotherapy drugs called fluoropyrimidines (this includes capecitabine and fluorouracil). Your doctor can tell you more.
- are pregnant or breastfeeding
- have any other serious medical condition or mental health problem that the trial team think could affect you taking part
Trial design
This is a phase 2 trial. The team need to find 45 people to take part.
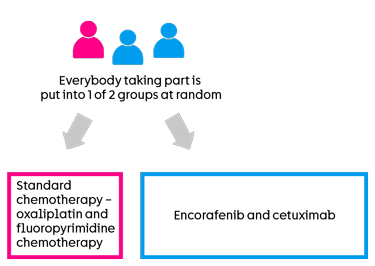
This is a randomised trial. A computer puts you into a treatment group. Neither you nor your doctor will be able to decide which group you are in.
There are 2 groups. You have 1 of the following treatments before surgery:
standard chemotherapy  – this is oxaliplatin and a type of
– this is oxaliplatin and a type of fluoropyrimidine chemotherapy 
- encorafenib and cetuximab
2 out of every 3 people have encorafenib and cetuximab. And 1 out of every 3 people have standard chemotherapy.
Encorafenib and cetuximab group (targeted cancer drugs)
You have 6 weeks of targeted cancer drug treatment before surgery. You have cetuximab as a drip into the bloodstream every 2 weeks for the 6 weeks. You take encorafenib as capsules once a day for 6 weeks.
When you finish the targeted treatment, you have no treatment for 3 to 4 weeks. You then have surgery to remove the cancer. The team talk to you about surgery, what it involves and how long you’ll stay in hospital for afterwards. It will take about a month or two for you to get better after surgery.
You then see your team to talk about whether you need more treatment.
Standard chemotherapy
You have 6 weeks of chemotherapy before surgery. You have chemotherapy as  .
.
You have one of the following:
Your doctor will talk to you about which treatment option they think will work best for you.
Oxaliplatin and capecitabine (OxCap)
You have oxaliplatin as a drip into the bloodstream every 3 weeks for the 6 weeks. You have capecitabine as tablets twice a day for two weeks, then a break for one week.
You then start the next treatment cycle.
Oxaliplatin and fluouracil (5FU) with folinic acid (FOLFOX)
Oxaliplatin and fluouracil are chemotherapy drugs. Folinic acid helps the fluouracil work better. You have all the drugs as a drip into the bloodstream.
You have treatment through a long plastic tube that goes into a large vein in your chest. The tube stays in place throughout the course of treatment. The tube will be one of the following:
- central line
- PICC line
- portacath
You have FOLFOX once every 2 weeks for the 6 weeks.
You have oxaliplatin on day 1 of your cycle. You have folinic acid at the same time as oxaliplatin. You have fluorouracil as an injection into your vein, then you start fluorouracil as an infusion over 46 hours. You have a small portable pump that gives you the chemotherapy.
You can keep the pump in a small bag, or a bag on a belt (like a bum bag). You’ll need to go back to the hospital after the second day of treatment, to have the pump disconnected. Or sometimes a chemotherapy nurse may be able to do this at your home.
After chemotherapy
When you finish chemotherapy, you have no treatment for 4 to 8 weeks. You then have surgery to remove the cancer. The team talk to you about surgery, what it involves and how long you’ll stay in hospital for afterwards. It will take about a month or two for you to get better after surgery.
You then see your team to talk about whether you need more treatment.
Samples for research
The trial team look at tissue collected from the  you had at diagnosis. You don’t need to give a new sample for this. They do some tests on the sample. They want to find out if the cells have:
you had at diagnosis. You don’t need to give a new sample for this. They do some tests on the sample. They want to find out if the cells have:
- gene changes (mutations) that usually help fix mistakes when DNA is copied in a cell. This is called mismatch repair or MMR. This might help doctors predict how well chemotherapy will work.
- the BRAF
gene  change (mutation) called BRAFV600E. The cells need this change for the targeted treatment to work.
change (mutation) called BRAFV600E. The cells need this change for the targeted treatment to work.
The trial team also ask if they can use the sample for future research to learn more about bowel cancer. You can say no and still take part in the trial.
The trial team ask to collect tissue samples when you have surgery. This is to see how well the treatment has worked. They also ask to take extra blood samples. Where possible you have these at the same time as routine blood samples.
The extra blood samples are to look at the amount of cancer cells in your blood. This is called circulating tumour DNA ( ). The trial team aim to understand more about:
). The trial team aim to understand more about:
- bowel cancer
- when treatment is likely to work well
- how to monitor the cancer after surgery
You can say no to the extra samples and still take part in the trial.
Quality of life
The trial team ask you to fill out a questionnaire:
- before you start treatment
- at set times during and after treatment
The questionnaire asks about side effects and how you’ve been feeling. This is called a quality of life study. You fill in the questionnaire in the hospital, or at home if you have finished treatment.
Hospital visits
You see the doctor and have tests before you can take part. These include:
- blood tests
- a
physical examination 
- a pregnancy test - if there is any chance you might be pregnant
- a
CT scan  of your chest, tummy (abdomen) and
of your chest, tummy (abdomen) and pelvis 
- a colonoscopy
You have a heart trace ( ) if you are having encorafenib and cetuximab.
) if you are having encorafenib and cetuximab.
You might have had some of these tests at diagnosis. Talk to the trial team about which tests you still need.
You have a check up and blood tests before each cycle of targeted treatment or chemotherapy.
When you finish your trial treatment you have a check up a few weeks later to see how you are getting on before surgery.
As part of the trial, you may have a CT scan of your chest, tummy (abdomen) and pelvis after your trial treatment and before surgery.
You have a heart trace (ECG) after a month of targeted treatment. This is the same as if you were having encorafenib not on the trial.
Your hospital doctor will talk with you about chemotherapy after surgery once you have recovered from the operation. They will answer any questions you have.
Follow up
You see the trial team after surgery to see how you are getting on. This is the same if you have chemotherapy after surgery or not. This is called follow up.
When you finish treatment, you have routine check ups. These include:
- blood tests every 6 months for 3 years
- a CT scan every year for 3 years
Everyone has a CT scan 3 years after joining a treatment group in the trial.
Side effects
The trial team monitor you during treatment and afterwards. Contact your advice line or tell your doctor or nurse if any side effects are bad or not getting better.
The trial doctor will talk to you about all the possible side effects of treatment. You will have a chance to ask them any questions you may have.
The most common side effects of encorafenib and cetuximab are:
- skin reactions
- tiredness (fatigue) or weakness
- feeling or being sick
- diarrhoea or constipation
- tummy (abdominal) pain
- joint or muscle pain
- loss of appetite
We have more information, including possible side effects, about:
Location
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Jenny Seligman
Supported by
Yorkshire Cancer Research
University of Leeds
Pierre Fabre
Merck
The Beatson West of Scotland Cancer Centre
University of Birmingham
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040