Please note
This trial is no longer recruiting patients. We hope to add results when they are available.
Head and neck cancers, Laryngeal cancer, Mouth and oropharyngeal cancer, Pharyngeal cancer, Secondary cancers
Closed
Phase 3
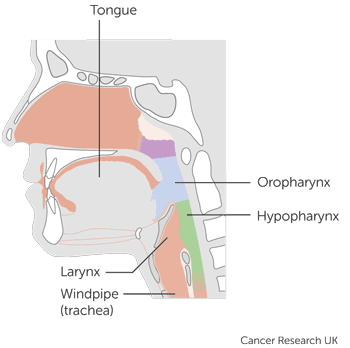
This trial is for people with stage 3 or stage 4a of the:
mouth and oropharynx
larynx
Stage 3 or stage 4a means one of the following:
the cancer is bigger than 4cm but has not spread into any lymph nodes or any other part of the body
the cancer is any size but has spread to one lymph node on the same side of the neck as the cancer. And the lymph node is no more than 3cm across
the cancer has grown through the tissues around the lips and mouth
Everyone taking part is going to have treatment for head and neck cancer for the first time.
Recruitment start: 10 January 2019
Recruitment end: 22 September 2023
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Dr Mary Lei
Merck Sharp & Dohme Ltd
Last reviewed: 15 Nov 2023
CRUK internal database number: 15800