A trial looking at pegylated interferon to treat melanoma (EORTC 18081)
Cancer type:
Status:
Phase:
This trial looked at pegylated interferon (also called peginterferon) to treat melanoma.
The trial was for people with an ulcerated melanoma that had grown into the skin ( ). There were no signs that their melanoma had spread to nearby
). There were no signs that their melanoma had spread to nearby  or elsewhere in the body.
or elsewhere in the body.
This trial was open for people to join between 2013 and 2017. The team published the results in 2020.
More about this trial
Doctors treat melanoma by removing it with surgery. An ulcerated melanoma breaks the skin covering it. There is a high chance of ulcerated melanoma coming back or spreading to another part of the body.
When this trial was done, there was no further  after surgery to remove ulcerated melanoma. Researchers wanted to find out if peginterferon could help people in this situation.
after surgery to remove ulcerated melanoma. Researchers wanted to find out if peginterferon could help people in this situation.
Interferon boosts the body’s  . Interferon is already used to treat some people with melanoma.
. Interferon is already used to treat some people with melanoma.
Peginterferon is the  form of interferon. It stays in the body for a longer period of time.
form of interferon. It stays in the body for a longer period of time.
To find out if peginterferon did help, the researchers wanted to compare people who had peginterferon after surgery with those who didn’t.
The aims of this trial were to find out:
- how well peginterferon works for people with ulcerated melanoma
- how safe peginterferon is
Summary of results
The trial showed that peginterferon could be a treatment option for people with ulcerated melanoma.
This trial closed early due to poor recruitment.
About this trial
This was a phase 3 international trial. Worldwide 112 people took part with 21 from the UK. Everyone had surgery to remove their cutaneous melanoma.
It was a randomised trial. There were 2 groups. Neither the people taking part nor their doctor chose which group they were in. After surgery:
- 56 people had peginterferon
- 56 people didn’t
People had treatment for up to 2 years as long as it was working and the side effects weren’t too bad.
Results
Of the 56 people in the peginterferon group, 54 had at least 1 injection. The team looked at how many of these 54 people completed treatment and why they stopped. They found that:
- 16 people completed the 2 year treatment
- 2 people stopped because the melanoma came back
- 23 people stopped due to side effects
- 7 people decided to stop their treatment
- 6 people stopped treatment for other reasons
The team looked at the most common side effects that caused people to stop treatment. They were:
- tiredness (fatigue)
- high temperature (fever)
Of the 56 people who didn’t have peginterferon:
- 33 people took part in the trial for the 2 years
- 12 people left the trial early because the melanoma came back
- 11 people left the trial early for other reasons
After a  follow up of just under 3½ years (3.4 years) the number of people in each group whose melanoma had come back was:
follow up of just under 3½ years (3.4 years) the number of people in each group whose melanoma had come back was:
- 13 people who had peginterferon
- 17 people who didn’t have peginterferon
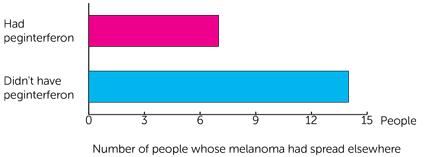
The total number of people whose melanoma had spread elsewhere in the body was 21.
- 7 people were from the group who had peginterferon.
- 14 people were from the group who didn’t have peginterferon.
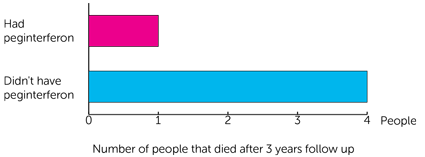
The team also looked at the number of people who had died. The total was 5 people. This was because their melanoma got worse.
- 1 person had peginterferon.
- 4 people didn’t have peginterferon
Side effects
The most common side effects of peginterferon included:
- a drop in blood cells causing an increased risk of infection bruising and bleeding or breathlessness
- diarrhoea
- feeling or being sick
- loss of hair
- sore muscles
- loss of appetite or weight loss
- a high level of a fat called triglyceride in the blood
- headache
- changes to how the liver works
- problems with sleep, anxiety or depression
- reaction at the injection site
- flu-like symptoms
- fever
- tiredness (fatigue)
- chills
- high blood pressure
Conclusion
The trial team concluded that peginterferon was an option for people with ulcerated melanoma that had not spread to the lymph nodes or elsewhere in the body. These results may be useful for countries where new treatments aren’t available. For example, new treatments such as immunotherapy drugs.
Where this information comes from
We have based this summary on information from the research team. The information they sent us has been reviewed by independent specialists ( ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Poulam Patel
Doctor Alessandro Testori
Supported by
Experimental Cancer Medicine Centre (ECMC)
European Organisation for Research and Treatment of Cancer (EORTC)
NIHR Clinical Research Network: Cancer
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040