A trial looking at further treatment to the armpit after surgery to remove breast cancer (ATNEC)
Cancer type:
Status:
Phase:
This trial is looking at the need to treat the  in the armpit after having chemotherapy followed by surgery for breast cancer.
in the armpit after having chemotherapy followed by surgery for breast cancer.
More about this trial
The  for breast cancer is chemotherapy to shrink the cancer before surgery. After surgery you have further treatment to the lymph glands in the armpit on the side where the cancer was. This is to make sure that there isn’t any cancer in the lymph nodes of the armpit and reduce the risk of the cancer coming back.
for breast cancer is chemotherapy to shrink the cancer before surgery. After surgery you have further treatment to the lymph glands in the armpit on the side where the cancer was. This is to make sure that there isn’t any cancer in the lymph nodes of the armpit and reduce the risk of the cancer coming back.
You have either more surgery to remove the lymph nodes in the armpit or radiotherapy to the armpit. As with all treatments there are side effects such as:
- a stiff shoulder
- numb hand or arm
- pain in the hand or arm
Some people might develop a problem with the fluid draining from their arm and this causes swelling of the hand and or arm. This is 
We know that having chemotherapy before surgery can completely get rid of the cancer in the lymph glands for between 40 to 70 people out of every 100 (40% to 70%).
In this trial the researchers want to find out whether only having chemotherapy before surgery is just as good for these people. If so they wouldn’t need to have treatment to their armpit and this might reduce the risk of side effects.
The aims of this trial are to find out:
- whether not treating the lymph glands in the armpit after chemotherapy and surgery is as good as the current treatment. This is for people who don’t have cancer in the lymph nodes of their armpit following chemotherapy.
- whether it reduces the risk of getting lymphoedema and other side effects
- how it affects the
quality of life 
Who can enter
The following bullet points are a summary of the entry conditions for this trial. Talk to your doctor or the trial team if you are unsure about any of these. They will be able to advise you.
Who can take part
You may be able to join this trial if all of the following apply. You:
- have cancer that hasn’t spread and there are cancer cells in 1 to 3 lymph nodes in the armpit (cT1 to T3, N1, M0)
- have had a sample tissue (
biopsy  ) of lymph nodes in the armpit confirming cancer cells in the nodes
) of lymph nodes in the armpit confirming cancer cells in the nodes - have had tests to find out whether your cancer is
hormone sensitive  or not
or not - have had chemotherapy before surgery to shrink the cancer
- have had a scan of your armpit to assess how well the chemotherapy has worked. This can be an ultrasound, an MRI scan, a PET scan or CT scan.
- have had 2 or 3 lymph nodes removed including a
sentinel lymph node  after chemotherapy. Your doctor will know about this.
after chemotherapy. Your doctor will know about this. - are at least 18 years old
Who can’t take part
You cannot join this trial if any of these apply. You:
- have
invasive breast cancer  in both breasts at the same time
in both breasts at the same time - had a sentinel lymph node removed before having chemotherapy to shrink the cancer
- have had previous surgery to the armpit on the same side as your breast cancer
- have remaining cancer in the in the lymph nodes after your chemotherapy
- had another cancer or had another cancer within the past 5 years apart from
non melanoma skin cancer  ,
, early stage  melanoma or
melanoma or in situ carcinoma  of the cervix or breast
of the cervix or breast
Trial design
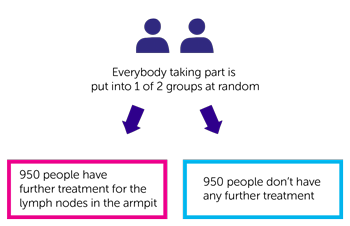
This is a phase 3 trial. The team need 1,900 people to join.
It is a randomised trial. You go into 1 of 2 treatment groups. Neither you nor your doctor can choose which group you go into. The groups are:
- 950 people have further treatment for the lymph nodes in the armpit
- 950 don’t have any further treatment
Further treatment is either more surgery to remove all the lymph nodes or radiotherapy to the lymph nodes. Your doctor will tell you about both treatments and then you and your doctor can choose which is best for you.
Quality of life
You will fill in one questionnaire booklet when you join the trial and then one questionnaire booklet every year for 5 years. The questions ask about:
- your quality of life
- what you are able to do
- any feelings of worry you might have
These are quality of life questionnaires.
Research samples
The team ask to collect and store any extra tissue left from when you have your surgery and tissue samples ( ).
).
You don’t have to agree to this. You can still take part in the trial.
Hospital visits
There are no extra hospital visits if you take part in the trial.
Side effects
The trial team monitor you during treatment and afterwards. Contact your advice line or tell your doctor or nurse if any side effects are bad or not getting better.
The possible side effects of having treatment to the armpit include:
- shoulder stiffness
- numb arm
- swelling of hand and or the arm (lymphoedema)
Your doctor or a member of the trial team will talk to you about these side effects before you agree to join the trial.
Location
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Associate Professor Amit Goyal
Supported by
NIHR Clinical Research Network: Cancer
NIHR Health Technology Assessment (HTA) programme
NCRI Radiotherapy Trials QA Group (RTTQA)
Warwick Clinical Trials Unit
University Hospitals of Derby and Burton NHS Foundation Trust
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040