A trial looking at durvalumab with or without tremelimumab for non small cell lung cancer that has spread (ARCTIC)
Cancer type:
Status:
Phase:
This trial compared durvalumab alone, or alongside tremelimumab, with usual treatment for people with non small cell lung cancer.
It was for people whose cancer had grown into surrounding tissues or had spread to another part of the body. This is locally advanced or advanced cancer.
This trial was open for people to join between 2015 and 2016. The team published the results in 2020.
More about this trial
There are a number of different treatments for non small cell lung cancer that has spread. But sometimes these stop working and the cancer comes back. When this trial was done, doctors wanted to improve treatment for people in this situation.
In this trial they looked at 2 drugs called durvalumab and tremelimumab. They are both immunotherapies and work by helping the  to attack cancer cells. They function in slightly different ways.
to attack cancer cells. They function in slightly different ways.
The main aims of the trial were to:
- find out how well the combination of durvalumab and tremelimumab works compared with usual treatment
- find out how well durvalumab works on its own compared with usual treatment
- learn more about the side effects
Summary of results
The trial team found that the combination of durvalumab and tremelimumab didn’t improve treatment outcome for advanced NSCLC any more than the usual treatment.
There was a slight increase in how long people who had durvalumab on its own lived. But the difference isn’t big enough for the research team to say for sure that it’s because of durvalumab treatment.
Trial design
This was a phase 3 trial. It took place worldwide.
The team had hoped to find about 1,560 people to join. But it was difficult to find enough people. This was because newer treatments were approved for NSCLC at the start of the trial.
595 people in total took part.
The team looked at a sample of cancer tissue ( from everyone in the trial. This was a sample that people gave when their cancer was first diagnosed.
from everyone in the trial. This was a sample that people gave when their cancer was first diagnosed.
The team tested the sample for a protein called PD-L1. Cancer that has large amounts of PD-L1 is PD-L1 positive. Cancer that has small amounts of PD-L1 is PD-L1 negative.
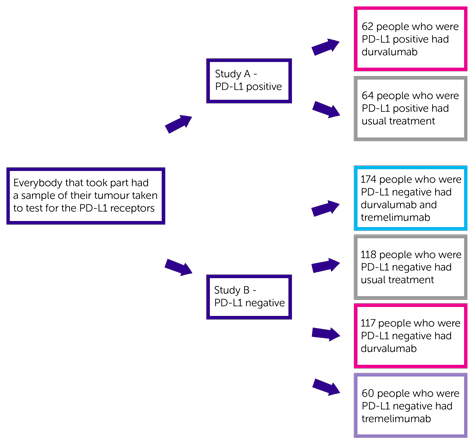
This trial had 2 separate parts. People who had PD-L1 positive cancer joined study A. People who had PD-L1 negative cancer joined study B.
People had one of the following treatments:
- durvalumab
- tremelimumab
- durvalumab and tremelimumab
- usual treatment
The usual treatment options for study A and study B included 1 of the following:
- a chemotherapy drug called gemcitabine
- a chemotherapy drug called vinorelbine
- a targeted drug called erlotinib
The doctor recommended the best suitable standard treatment option.
Treatment groups
Study A was for people whose cancer was PD-L1 positive. 126 people joined study A. There were 2 treatment groups. People were put into a treatment group at random:
- 62 people who were PD-L1 positive had durvalumab
- 64 people who were PD-L1 positive had usual treatment
Study B was for people whose cancer was PD-L1 negative. 469 people joined study B. There were 4 treatment groups. People were put into a treatment group at random:
- 174 people who were PD-L1 negative had durvalumab and tremelimumab
- 118 people who were PD-L1 negative had usual treatment
- 117 people who were PD-L1 negative had durvalumab
- 60 people who were PD-L1 negative had tremelimumab
Results for study A
The trial team looked at how well treatment worked. To do this they looked at how long before the cancer started to grow again after starting treatment. They found this was about:
- 3.8 months for those who had durvalumab
- 2.2 months for those who had usual treatment
They also looked at how long people lived after starting treatment. They found it was:
- 11.7 months for those who had durvalumab
- 6.8 months for those who had usual treatment
Results for study B
The team looked at how well treatment worked. They were mainly interested in the combination of durvalumab and tremelimumab compared with usual treatment.
The team looked at how long before the cancer started to grow again after starting treatment. They found it was:
- 3.5 months for those who had durvalumab and tremelimumab or
- usual treatment
- 3.1 months for those who had durvalumab alone
- 2.1 months for those who had tremelimumab alone
So they didn’t find a difference between people who had the combination of durvalumab and tremelimumab and those who had usual treatment.
The team also looked at how long people lived after starting treatment. They found this was:
- 11.5 months for those who had durvalumab and tremelimumab
- 8.7 months for those who had usual treatment
- 10 months for those who had durvalumab
- 6.9 months for those who had tremelimumab
Although these numbers look different. The difference isn’t big enough for the research team to say for sure that it’s because of the different treatments. It could be due to chance.
Side effects
Most people who took part in this trial had at least 1 side effect. Most were mild or didn’t last long. More people in the usual treatment group had side effects that were severe.
Study A
The trial team looked at all the side effects. We have included those that they think were related to treatment.
The severe side effects of usual care were:
- a drop in white blood cells causing an increased risk of infection
- a drop in red blood cells causing an increased risk of breathlessness and tiredness (
anaemia  )
) - a high temperature (febrile neutropenia) and low levels of
white blood cells 
The most common side effects of usual care were:
- a drop in red blood cells (anaemia)
- loss of appetite
The most common side effects of durvalumab included:
- loss of appetite
- tiredness (fatigue)
Study B
The trial team looked at all the side effects. We have included those that they think were related to treatment.
People who had durvalumab on its own had more side effects that were mild.
The side effects of usual care were similar to those found in study A.
The most common severe side effects of:
- durvalumab and tremelimumab were diarrhoea and inflammation of the lung tissue (
pneumonitis  )
) - durvalumab alone were fatigue and shortness of breath
- tremelimumab alone was diarrhoea
In study B, one person died during treatment with durvalumab. And one person died during treatment with tremelimumab.
Conclusion
Study A was for people who had PD-L1 positive NSCLC. There was a slight increase in how long people who had durvalumab only lived compared with usual treatment. The difference between the groups wasn’t big enough to say for sure.
Study B was for people with PD-L1 negative NSCLC. In study B, the team concluded that the combination of durvalumab and tremelimumab was no more effective than usual treatment. It didn’t increase the length of time before the cancer started to grow again or how long people lived compared with usual treatment.
Even so, all trial results help doctors and researchers understand more about different cancers and the best way to treat them.
There are other trials looking at durvalumab on its own for people with NSCLC. And there are also trials looking at durvalumab in combination with tremelimumab.
Where this information comes from
We have based this summary on information from the research team. The information they sent us has been reviewed by independent specialists ( ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr David Planchard
Supported by
AstraZeneca
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040