Please note
This trial is no longer recruiting patients. We hope to add results when they are available.
Head and neck cancers, Laryngeal cancer, Mouth and oropharyngeal cancer, Pharyngeal cancer
Closed
Phase 3
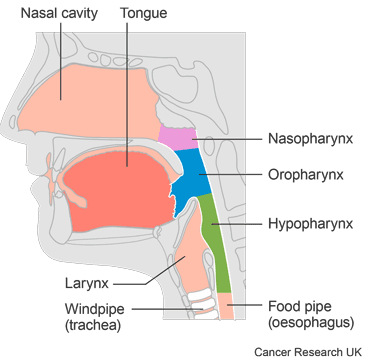
This trial is for people with cancer of the:
mouth (oral cavity)
oropharynx
hypopharynx
larynx
It is for people who have completed initial treatment for their head and neck cancer and have a high risk of the cancer coming back or getting worse.
Recruitment start: 12 July 2018
Recruitment end: 14 February 2020
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Professor Kevin Harrington
Roche
Last reviewed: 18 Feb 2020
CRUK internal database number: 16242