A trial comparing different ways of deciding on treatment after surgery for breast cancer (MINDACT)
Cancer type:
Status:
Phase:
This trial looked at whether a group of 70 genes could help doctors work out who needed to have more treatment after surgery for breast cancer.
It is also looked at different:
- combination of chemotherapy drugs (with or without an
anthracycline drug  ) for women who were at high risk of breast cancer coming back
) for women who were at high risk of breast cancer coming back - types of hormone therapy for women who had hormone receptor positive breast cancer
Cancer Research UK supported this trial.
More about this trial
Surgery is usually the first treatment for early breast cancer that can be removed with surgery. As well as removing the cancer, the surgeon also removes some  from under your arm. The lymph nodes and the cancer from your breast are looked at under the microscope.
from under your arm. The lymph nodes and the cancer from your breast are looked at under the microscope.
Doctors work out how likely it’s for the cancer to come back by looking at:
- the appearance of the cancer cells and how fast they grow (the grade)
- the size of the cancer
- if there are cancer cells in the lymph nodes
- if the cancer cells have hormone receptors and
HER2 receptors 
They offer you chemotherapy after surgery to reduce this risk if they think it is high. They advise you to have hormone therapy if there are hormone receptors on the cancer cells. This is called adjuvant treatment.
In this trial researchers looked at a new way of testing breast cancer cells to see who should have adjuvant treatment. The test involved looking at 70  in the cancer cells to see which ones were active. This method is called ‘genomics’.
in the cancer cells to see which ones were active. This method is called ‘genomics’.
It was not a test to see if breast cancer might run in the family. It only looked at the genes in the cancer cells. The researchers wanted to find out if this test could predict if you might develop breast cancer in another part of your body later on.
Doctors often use a drug called doxorubicin, or a taxane drug (paclitaxel or docetaxel) for adjuvant chemotherapy. These can cause long term side effects, including damage to the heart.
Other chemotherapy drugs that have fewer side effects might be used to treat breast cancer. So, women who had chemotherapy were asked to join another part of the trial that compared different combinations of chemotherapy drugs.
The trial also looked at different types of hormone therapy for women who had hormone receptor positive breast cancer.
Doctors wanted to find if it was better to have tamoxifen followed by letrozole or to have just letrozole.
The aims of the trial were to:
- see if genomic test on cancer cells were better for deciding who needed to have adjuvant treatment than current ways of testing
- find which combination of chemotherapy drugs worked best after surgery for early breast cancer
- see if it was better to have had tamoxifen and letrozole or letrozole alone after surgery for hormone receptor positive breast cancer
Summary of results
The following are the first findings from the trial team looking at the genomic test only.
The team found that using the genomic test alongside the current clinical method provided valuable information as to who might benefit from having adjuvant chemotherapy.
This was a phase 3 trial.
All of the 6,693 women who joined the trial had their risk of breast cancer coming back assessed using the current (clinical) method and the genomic test. Of these women:
- 2,634 women had a low clinical risk and a low genomic risk
- 690 women had a low clinical risk and a high genomic risk
- 1,497 women had a high clinical risk and a low genomic risk
- 1,873 women had a high clinical risk and a high genomic risk
Women who had a low clinical risk and low genomic risk were advised not to have chemotherapy.
Women who had a high clinical risk and high genomic risk were advised to have chemotherapy.
Women whose clinical risk and genomic risk didn’t match were put into 1 of 2 groups by a computer. They were randomised to have chemotherapy or not.
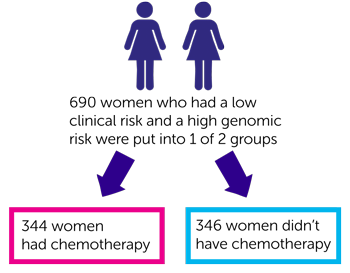
For the 690 women who had a low clinical risk and a high genomic risk:
- 344 women had chemotherapy
- 346 women didn’t have chemotherapy
After 5 years of follow up the researchers looked at how many women were alive and their cancer had not spread. They found this was:
- 96 out of every 100 women (95.8%) who had chemotherapy
- 95 out of every 100 women (95%) who didn’t have chemotherapy
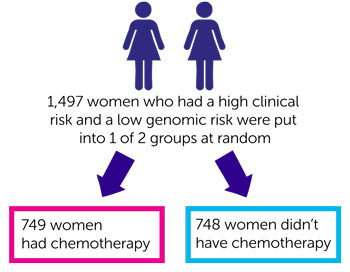
The 1,497 women who had a high clinical risk and a low genomic risk were also put into 1 of 2 groups at random:
- 749 women had chemotherapy
- 748 women didn’t have chemotherapy
After 5 years of follow up the researchers looked at how many women were alive and their cancer had not spread. They found this was:
- 95 out of every 100 women (94.4%) who had chemotherapy
- 96 out of every 100 women (95.9%) who didn’t have chemotherapy
The team calculated how many high clinical risk women might not have needed chemotherapy if genomic risk was used as a guide for further treatment. They said it was 46 out of every 100 women (46.2%).
The team concluded for women with a high clinical risk of their cancer coming back the need for chemotherapy might be reduced if genomic risk was also considered.
The team are continuing to follow up these women. When more results become available this summary will be updated.
We have based this summary on information from the research team. The information they sent us has been reviewed by independent specialists ( ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Alastair M Thompson
Supported by
Cancer Research UK
Breast International Group
Experimental Cancer Medicine Centre (ECMC)
European Organisation for Research and Treatment of Cancer (EORTC)
NIHR Clinical Research Network: Cancer
Other information
This is Cancer Research UK trial number CRUK/07/018.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



