A study looking at vaccines for prostate cancer (VANCE01)
Cancer type:
Status:
Phase:
This study looked at two vaccines called ChAdOx1.5T4 and MVA.5T4, with or without chemotherapy.
It was for men who were due to have surgery to remove their prostate cancer or who were having active surveillance. Active surveillance means having regular tests and check ups, and having treatment if the cancer starts to grow.
The study was open for people to join between 2015 and 2017. The team published the results in 2020.
More about this trial
Vaccines can encourage the immune system to recognise and attack cancer cells. This is called an immune response.
5T4 is a protein found on prostate cancer cells. This study looked at 2 vaccines that can target the 5T4 protein:
- chimpanzee adenovirus (ChAdOx1.5T4)
- modified vaccinia ankara (MVA.5T4)
Researchers hoped that these vaccines would help the immune system target the 5T4 protein and kill the prostate cancer cells.
They also wanted to see if having low dose chemotherapy helps the vaccines to work better. So some people taking part had cyclophosphamide as well.
Those who had chemotherapy took cyclophosphamide tablets for a week before each vaccine dose.
The main aims of the study were to find out:
- whether the vaccines cause an immune response
- what the side effects are
Summary of results
The research team found that the two vaccines can cause an immune response.
Study design
This study was for men with prostate cancer that:
- was due to be removed with surgery, or
- didn’t need treatment straight way but was being monitored (active surveillance)
There were 8 treatment groups in total.
The men due to have surgery were put into 1 of 6 groups at random:
Group 1 had ChAdOx1.5T4 and then two doses of MVA.5T4, with 4 weeks between each dose. They had surgery to remove their prostate 12 weeks after starting treatment.
Group 2 had ChAdOx1.5T4 and then two doses of MVA.5T4, with 4 weeks between each dose. They also had cyclophosphamide tablets for 1 week before each vaccine dose. They had surgery to remove their prostate 12 weeks after starting treatment.
Group 3 had 3 doses of MVA.5T4, with 4 weeks between each dose. They had surgery to remove their prostate 12 weeks after starting treatment.
Group 4 had 3 doses of MVA.5T4, with 4 weeks between each dose. They also had cyclophosphamide tablets for 1 week before each vaccine dose. They had surgery to remove their prostate 12 weeks after starting treatment.
Group 5 had ChAdOx1.5T4 and then MVA.5T4 one week later. They had surgery to remove their prostate 4 weeks after starting treatment.
Group 6 had ChAdOx1.5T4 and then MVA.5T4 one week later. They also had cyclophosphamide tablets for 1 week before each vaccine dose. They had surgery to remove their prostate 4 weeks after starting treatment.
The men who were having active surveillance were put into 1 of 2 groups at random:
Group 7 had ChAdOx1.5T4 and then MVA.5T4 one week later. They had a biopsy of their prostate 10 weeks after starting treatment.
Group 8 had ChAdOx1.5T4 and then MVA.5T4 one week later. They also had cyclophosphamide tablets for 1 week before each vaccine dose. They had a biopsy of their prostate 10 weeks after starting treatment.
Results
A total of 40 men with prostate cancer joined this study. There were between 2 and 8 people in each treatment group.
The research team looked at how many people’s immune system responded to the vaccine. They were able to analyse this for 39 people who took part.
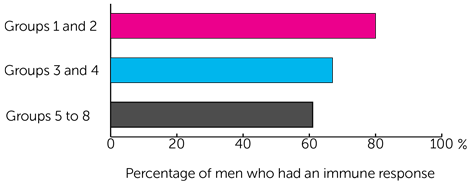
The results showed that the immune system responded to the vaccine in:
- 4 out of 5 men (80%) in groups 1 and 2 - who had ChAdOx1.5T4 and then 2 doses of MVA.5T4
- 4 out of 6 men (67%) in groups 3 and 4 - who had 3 doses of MVA.5T4
- 17 out of 28 men (61%) in groups 5 to 8 - who had ChAdOx1.5T4 and then MVA.5T4 a week later
The immune response wasn’t any bigger in people who had cyclophosphamide, compared to those who didn’t.
Side effects
Most men had at least 1 side effect. But nearly all the side effects people had (92%) were mild or didn’t last long. They included:
- mild or moderate pain at the injection site
- fever
- aching muscles
- extreme tiredness (fatigue)
Conclusion
The research team concluded that ChAdOx1.5T4 and MVA.5T4 vaccines can cause an immune response in people with prostate cancer. They also found the vaccines didn’t cause too many side effects.
They suggest more work is done to find out if they are likely to be a useful treatment for prostate cancer.
These results led to a trial called ADVANCE. This was for men with more advanced prostate cancer. The men who took part had the vaccine alongside standard treatment. We hope to include the results of ADVANCE on our website soon.
More detailed information
There is more information about this research in the reference below.
Please note, this article is not in plain English. It has been written for health care professionals and researchers.
Safety and immunogenicity of novel 5T4 viral vectored vaccination regimens in early stage prostate cancer: a phase I clinical trial
F Cappuccini and others
The Journal for ImmunoTherapy of Cancer, 2020. Volume 8, issue 1, pages 1 to 13.
Where this information comes from
We have based this summary on the information in the article above. This has been reviewed by independent specialists ( ) and published in a medical journal. We have not analysed the data ourselves. As far as we are aware, the link we list above is active and the article is free and available to view.
) and published in a medical journal. We have not analysed the data ourselves. As far as we are aware, the link we list above is active and the article is free and available to view.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Freddie Hamdy
Supported by
Experimental Cancer Medicine Centre (ECMC)
European Commission
NIHR Clinical Research Network: Cancer
University of Oxford
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040