A trial looking at surgery for people with kidney cancer that has spread (SURTIME; EORTC 30073)
Cancer type:
Status:
Phase:
This trial looked at the best time for people with kidney cancer that has spread to another part of the body to have surgery. It was supported by Cancer Research UK.
More about this trial
This trial was for people with a type of kidney cancer called clear cell kidney cancer. Doctors usually treat clear cell kidney cancer with surgery to remove the kidney (a nephrectomy), and sunitinib.
Sunitinib (Sutent) is a type of targeted cancer drug called a tyrosine kinase inhibitor (TKI). It blocks the signals that cells use to divide and grow.
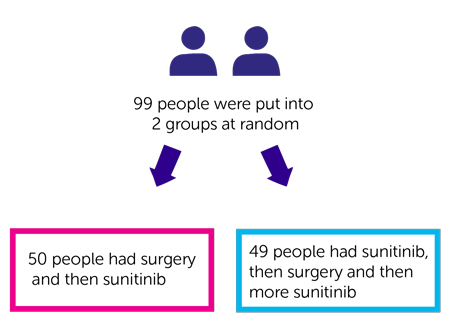
Researchers wanted to find out the best time to have surgery for this type of cancer. Some people in this trial had surgery first and then sunitinib. And some had sunitinib, then surgery, and then more sunitinib.
The main aim of this trial was to find out if it is better to have surgery or sunitinib first for clear cell kidney cancer that has spread.
Summary of results
- 50 people had surgery and then sunitinib
- 49 people had sunitinib, then surgery and then more sunitinib
- 46 people had surgery
- 40 people had sunitinib after their operation
- 48 people had some sunitinib to begin with
- 34 people had surgery
- 26 people had more sunitinib after their operation
- 21 out of 50 people (42%) who’d had surgery first
- 21 out of 49 people (43%) who’d had sunitinib first
- 35 out of 50 people (70%) who’d had surgery first
- 28 out of 49 people (57%) who’d had sunitinib first
- 14 out of 46 people (30%) who had surgery first
- 6 out of 34 people (18%) who had sunitinib first
- 18 out of 46 people (39%) who had surgery first
- 16 out of 34 people (47%) who had sunitinib first
 ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Axel Bex
Dr John Haanen
Supported by
Cancer Research UK
European Organisation for Research and Treatment of Cancer (EORTC)
Experimental Cancer Medicine Centre (ECMC)
NIHR Clinical Research Network: Cancer
Pfizer
Wales Cancer Trials Unit
Other information
This is Cancer Research UK trial number CRUK/10/050.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040