A trial looking at exercise and healthy eating for women with breast cancer (B-AHEAD)
Cancer type:
Status:
Phase:
This trial compared 3 different ways of promoting a healthy lifestyle in women who have breast cancer.
This trial was open to women to join between 2008 and 2010.
A poster of the results was presented at a conference in 2011.
More about this trial
- a healthy living booklet
- individual advice on healthy eating and physical activity followed by support at home by letter and phone
- individual advice on healthy eating and physical activity followed by weekly group exercise and dietary education classes
Summary of results
The trial team found that people were interested and motivated to make positive lifestyle changes after a diagnosis of breast cancer.
About this trial
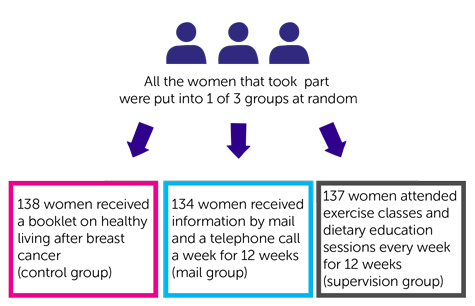
This was a phase 2 trial. 409 women agreed to join the trial.
It was a randomised trial. The women were put into 1 of 3 groups. Neither they nor their doctor chose which group they were in.
138 women received a booklet on healthy living after breast cancer. They were in the  .
.
Women in the other 2 groups received information about diet and exercise from a dietitian and an exercise specialist that was tailored for their individual needs.
After this initial meeting 134 women received information by mail and a telephone call a week for 12 weeks. The phone calls were to see how they were getting on with their plan. This was the mail group.
After their initial meeting the other 137 women attended exercise classes and dietary education sessions every week for 12 weeks. This was the supervision group.
Results
The team looked at the change in the women’s weight and body fat over a 6 month period. In each group they looked at women who were overweight and women who were of a healthy weight.
- a loss of half kilogram (0.5kg) for overweight women
- an increase of 1 kilogram (1kg) for women of a healthy weight
- a loss of just over 2 kilograms (2.1kg) for overweight women
- a loss of less than 1 kilogram (0.1kg) for women of a healthy weight
- a loss of just over 1 and a half kilograms (1.8kg) for overweight women
- a loss of less than 1 kilogram (0.2kg) for women of a healthy weight
- a loss of half a kilogram (0.5kg) for overweight women
- an increase of just over a half a kilogram (0.7kg) for women of a healthy weight
- a loss of just over 1 and a half kilograms (1.7kg) for overweight women
- a loss of just under half a kilogram (0.3kg) for women of a healthy weight
- a loss of 1 and a half kilograms (1.5kg) for overweight women
- a loss of just under half a kilogram (0.4kg) for women of a healthy weight
- the breast cancer had come back in 7 women
- 2 women developed another cancer
- 7 women had other health problems
- 5 women had family issues
- 4 of the women’s work and other time commitments meant they couldn’t continue
- 1 woman had regained weight
- 4 women had stress
- 3 women couldn’t be followed up (they were lost to follow up)
Of the 134 women who were in the mail group, 88 out of every 100 of them (88%) received their phone calls.
Conclusion
A high number of women agreed to take part in this trial and completed it. Because of this the trial team concluded that a trial like B-AHEAD was possible.
These are initial results. We will add further results as the trial team publishes them.
Where this information comes from
 ) but may not have been published in a medical journal. The figures we quote above were provided by the research team. We have not analysed the data ourselves.
) but may not have been published in a medical journal. The figures we quote above were provided by the research team. We have not analysed the data ourselves.Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Michelle Harvie
Supported by
Department of Health
NIHR Clinical Research Network: Cancer
NIHR Research for Patient Benefit (RfPB) Programme
The Genesis Appeal
University Hospital of South Manchester (UHSM)
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040