A trial of dovitinib for advanced womb cancer
Cancer type:
Status:
Phase:
This trial looked at dovitinib for womb (endometrial) cancer that had got worse despite treatment. It was for women who had already had one course of chemotherapy for advanced womb cancer.
More about this trial
 ) of the cancer to see if they had a changed (mutated) or normal gene.
) of the cancer to see if they had a changed (mutated) or normal gene. - see dovitinib helped women with either a normal or a changed FGFR2 gene
- learn more about the side effects
Summary of results
The trial team found that having dovitinib as the second treatment for advanced womb cancer worked a bit. But it didn’t work as well as they had hoped. And having the FGFR2 gene change didn’t affect how well treatment worked.
The researchers published these results in 2015.
About this trial
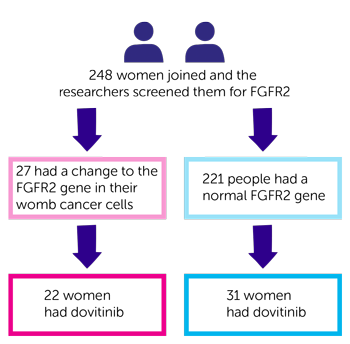
This was a phase 2 trial. 248 women joined and the researchers screened them for FGFR2. Of those:
- 27 had a change to the FGFR2 gene in their womb cancer cells
- 221 people had a normal FGFR2 gene
- 22 women with the FGFR2 gene change had dovitinib
- 31 women with a normal FGFR2 gene had dovitinib
- 7 out of the 22 women (32%) who had the FGFR2 gene change
- 9 out of the 31 women (29%) who had a normal gene
- 4.1 months in women who had the gene change
- 2.7 months in women who didn’t have the gene change
- diarrhoea
- feeling or being sick
- tiredness (fatigue)
- rash
- a blood clot on the lung
- being sick
- fluid loss (dehydration)
- diarrhoea
The trial team concluded that dovitinib worked a little bit for women with advanced womb cancer. But it didn’t work well enough to be looked at in a larger trial. Having a normal gene or a changed FGFR2 gene didn’t affect how well treatment worked.
This trial has increased knowledge about what works and what doesn’t work for advanced womb cancer.
We have based this summary on information from the research team. The information they sent us has been reviewed by independent specialists ( ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Rebecca Kristeleit
Supported by
Experimental Cancer Medicine Centre (ECMC)
NIHR Clinical Research Network: Cancer
Novartis
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040