A trial looking at durvalumab and tremelimumab for kidney cancer (RAMPART)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is looking at durvalumab and tremelimumab after having surgery to remove kidney cancer.
More about this trial
To treat kidney cancer doctors can remove part of the kidney or the whole kidney. After surgery you have regular  and clinic appointments to check whether your cancer is coming back. This is active monitoring.
and clinic appointments to check whether your cancer is coming back. This is active monitoring.
Durvalumab and tremelimumab are both immunotherapies. They work by helping your  attack cancer.
attack cancer.
In this trial some people:
- are actively monitored and don’t have either of the drugs in the study
- have durvalumab
- have durvalumab and tremelimumab
The aim of this trial is to find out if durvalumab on its own or in combination with tremelimumab can stop or delay kidney cancer coming back.
Who can enter
The following bullet points list the entry conditions for this trial. Talk to your doctor or the trial team if you are unsure about any of these. They will be able to advise you.
Who can take part
You may be able to join this trial if all of the following apply. You:
- have had surgery to remove your kidney cancer
- had surgery at least a month ago and no more than 3 months ago
- have a heart trace (
ECG  ) that shows your heart works well enough
) that shows your heart works well enough - have had a sample of tissue from your kidney cancer when you were diagnosed and are willing to give at least 1 fresh tissue sample (
biopsy  ) during the trial and you are also willing to give a blood sample
) during the trial and you are also willing to give a blood sample - are active and can look after yourself (performance status 0 or 1)
- have satisfactory blood test results
- are willing to use reliable contraception during treatment and for 6 months after if there is any chance you or your partner could become pregnant
- are at least 18 years old
Who can’t take part
Cancer related
You cannot join this trial if any of these apply. You:
- have had kidney cancer before
- still have some cancer left after surgery
- have cancer that has spread to another part of the body
- have had treatment for kidney cancer apart from surgery
- have ongoing side effects from treatment apart from hair loss (alopecia) and white patches on the skin (vitiligo)
- have had another cancer unless it was treated with the aim to cure, there has been no sign of it for 5 years or more and there is a low possibility of it coming back. This is apart from successfully treated
non melanoma skin cancer  , a type of melanoma called lentigo maligna and a
, a type of melanoma called lentigo maligna and a carcinoma in situ 
- have had cancer that spread to the tissue covering the brain and spinal cord
- have a lump on your lung that is 5mm or more across unless it is definitely non cancerous (benign). If you have many small lumps (less than 5mm across) you must have a scan that shows they are stable and not growing for at least 8 weeks
Medical conditions
You cannot join this trial if any of these apply. You:
- have had major surgery within 28 days of starting treatment. You might be able to join if you had local surgery treatment to relieve symptoms.
- are taking part in another clinical trial
- are taking medication that damps down your
immune system  within 14 days of starting treatment apart from nasal sprays, inhalers and a dose 10mg or less of steroids a day
within 14 days of starting treatment apart from nasal sprays, inhalers and a dose 10mg or less of steroids a day - have an
autoimmune disease  or inflammatory disease such as Chron’s, colitis, diverticulitis, systematic lupus erythematosus apart from hair loss, vitiligo, an underactive thyroid (hypothyroidism) on stable hormone replacements, skin condition not needing treatment that affects the whole body (systemic treatment), coeliac disease controlled with diet. You might be able to join if your disease hasn’t been active for the past 5 years.
or inflammatory disease such as Chron’s, colitis, diverticulitis, systematic lupus erythematosus apart from hair loss, vitiligo, an underactive thyroid (hypothyroidism) on stable hormone replacements, skin condition not needing treatment that affects the whole body (systemic treatment), coeliac disease controlled with diet. You might be able to join if your disease hasn’t been active for the past 5 years. - have HIV or another disease that affects your immune system
- have had an organ transplant including a bone marrow or stem cell transplant from another person (allogenic transplant)
- have another illness or condition that isn’t controlled for example COVID-19 or symptoms of COVID-19, infection, high blood pressure, chest pain (angina), an ongoing bleeding problem, stomach ulcer or inflammation of the gut, symptoms of congestive heart failure
- have active tuberculosis (TB)
- have active hepatitis B or hepatitis C
- have a lot of inflammation of the lungs or a lot of fibrosis tissue (fibrosis) on the lung
- are allergic to durvalumab, tremelimumab or any of their ingredients
- have another medical condition or mental health problem that the trial team or your doctor think could affect you taking part
Other
You cannot join this trial if any of these apply. You:
- are pregnant or breastfeeding
- have a
live vaccine  within 30 days of starting treatment. This does not include COVID-19 vaccines as they aren't live vaccines.
within 30 days of starting treatment. This does not include COVID-19 vaccines as they aren't live vaccines.
Trial design
This is an international phase 3 trial. The trial team need 1,750 people worldwide to join with 1,175 people from the UK.
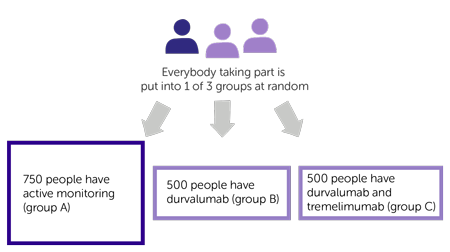
It is a randomised trial. There are 3 treatment groups. Neither you nor your doctor can choose which group you go into. For every 7 people who join:
- 3 people go into group A
- 2 people go into group B
- 2 people go into group C
The treatment groups are:
- 750 people are actively monitored (group A)
- 500 people having durvalumab (group B)
- 500 people having durvalumab and tremelimumab (group C)
Group A
You won’t have treatment. But have regular CT scans and clinic appointments to see how you are going.
Group B
You have durvalumab every 4 weeks for a year. You have durvalumab as a drip into a vein. It takes about an hour to have.
You have regular CT scans and clinic appointments to see how you are going.
Group C
You have durvalumab every 4 weeks for a year. On your 1st and 2nd treatment of durvalumab you also have tremelimumab.
You then continue with durvalumab on its own for a year. You have regular CT scans and clinic appointments to see how you are going.
Research samples
The team ask for a small piece of the cancer tissue that was taken out when you had surgery. You also give a blood sample before you start treatment. The team use these samples to find out:
- why some people have more side effects than others
- how well the treatment is working
- which people most likely benefit from having immunotherapy
You must agree to give these samples to take part in the trial.
Quality of life
You fill in 3 questionnaires:
- when you join the trial
- 3 times during the trial
The questions ask about:
- your general heath
- what you can do
- any symptoms or side effects you have
These are quality of life questionnaires.
You don’t have to agree to do the questionnaires if you don’t want to. You can still take part in the trial.
Hospital visits
You see the doctor to have tests before taking part. These tests include:
- a physical examination
- blood tests
- heart trace (
ECG  )
) - CT scan
Group A
During the trial you see the doctor at weeks 16 and 32 for a blood test and CT scan.
You are then seen by the doctor:
- every 3 months between year 1 and year 3
- then every 6 months to year 5
- then every year
Groups B and C
During treatment you see the doctor every 4 weeks for blood tests and to see how you are.
You are then seen by the doctor:
- every 3 months between year 1 and year 3
- then every 6 months to year 5
- then every year
Everyone has a CT scan at:
- 1 year
- then every 6 months to 3 years
- then at years 4, 5, 7 and 10
Side effects
The trial team monitor you during treatment and afterwards. Contact your advice line or tell your doctor or nurse if any side effects are bad or not getting better.
Durvalumab and tremelimumab can affect the immune system. This might cause inflammation in different parts of the body which can cause serious side effects. It could happen during treatment, or some months after treatment has finished. Rarely, these side effects could be life threatening.
If you have any of these side effects, you should tell the doctor or nurse as soon as possible that you are on or have been on an immunotherapy.
The most common side effects of taking durvalumab and tremelimumab include:
- cough that might produce sputum
- diarrhoea
- tummy (abdominal) pain
- rash or itching
- high temperature (fever)
- infection of the nose, throat or upper part of the chest
- feeling sick
- loss of appetite
- feeling tired and weak (fatigue)
- sore joints
There is a small chance you might have an allergic reaction to durvalumab or tremelimumab. This could happen hours or days after having treatment. Contact your doctor or the advice line if you have any of the following symptoms:
- swelling of the face or tongue
- trouble breathing or swallowing
We have more information about durvalumab.
Your doctor or a member of the trial team will talk about the possible side effects of treatment before you agree to take part.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor James Larkin
Supported by
Cancer Research UK
University College London (UCL)
Kidney Cancer UK
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040