A trial of bevacizumab and atezolizumab for pleural mesothelioma (BEAT-meso)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
More about this trial
 to recognise and kill cancer cells.
to recognise and kill cancer cells.- see if adding atezolizumab to chemotherapy and bevacizumab improves treatment
- find out more about the side effects
- find out more about
quality of life 
Who can enter
- have pleural mesothelioma that has spread into surrounding tissues or elsewhere in the body
- have pleural mesothelioma that your doctor can see on a scan
- have a tissue sample (
biopsy  ) available for the trial team to do some tests on
) available for the trial team to do some tests on - can carry out all your normal activities, apart from heavy physical work (performance status 0 or 1)
- have satisfactory blood test results
- are willing to use reliable contraception during treatment and for up to 6 months afterwards if there is any chance you or your partner could become pregnant
- are at least 18 years old
- are suitable to have surgery to remove the cancer
- have already had treatment for pleural mesothelioma
- have already had treatment with an immunotherapy drug including
interferon  or
or interleukin  in the last 4 weeks or the drugs haven’t completely cleared your body
in the last 4 weeks or the drugs haven’t completely cleared your body - have pleural mesothelioma that has grown into the large blood vessels in the chest
- are having or have had treatment that dampens down your
immune system  within 2 weeks of starting trial treatment (you may still take part if you are taking a low dose of the
within 2 weeks of starting trial treatment (you may still take part if you are taking a low dose of the steroid drug  prednisolone)
prednisolone) - have had a transplant with someone else’s cells (
allogeneic transplant  ) or an
) or an organ transplant  [Gloss/Organ transplant]
[Gloss/Organ transplant] - have high blood pressure or very high blood pressure in the past that affected brain function (hypertensive crisis)
- have had a problem with your blood vessels such as a bulge or swelling in the aorta (an aortic aneurysm) within 6 weeks of joining the trial
- have vomited blood with 1 month of joining the trial or had a significant bleeding disorder in the past
- are taking medication to thin the blood such as warfarin unless your blood clotting has been stable for the last 2 weeks
- have had aspirin within 10 days of starting trial treatment
- have had a minor surgical procedure within 7 days of starting bevacizumab
- have a wound or ulcer that hasn’t healed or an untreated bone fracture
- have had a severe allergic reaction to a
platinum drug  , pemetrexed, bevacizumab or atezolizumab in the past
, pemetrexed, bevacizumab or atezolizumab in the past - have moderate numbness or tingling in your hands or feet (
peripheral neuropathy  )
) - have HIV
- have an active hepatitis B or hepatitis C infection
- have any other medical condition or mental health problem that the trial team think would affect you taking part
- are pregnant or breastfeeding
- have had a live
vaccine  within 4 weeks of starting trial treatment
within 4 weeks of starting trial treatment
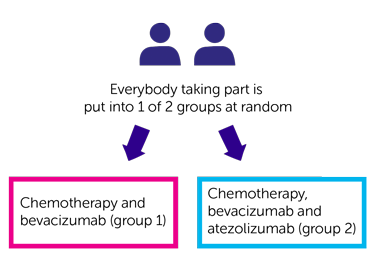
Trial design
- chemotherapy and bevacizumab (group 1)
- chemotherapy, bevacizumab, atezolizumab (group 2)
- carboplatin chemotherapy
- pemextred chemotherapy
- bevacizumab
- carboplatin chemotherapy
- pemextred chemotherapy
- bevacizumab
- atezolizumab
- see how well the treatment is working
- look for
biomarkers  to predict who will benefit from treatment
to predict who will benefit from treatment
Hospital visits
- physical examination
- blood samples
- urine samples
heart trace 
- CT scan
Side effects
- you have severe side effects
- your side effects aren’t getting any better
- your side effects are getting worse
- fever
- feeling or being sick
- tiredness (fatigue) or lack of energy
- itchy skin or skin rash
- diarrhoea
- joint pain, muscle or bone pain
- loss of appetite
- shortness of breath
- urine infections
- cough
- high blood pressure
- numbness or tingling in the hands or feet
- an increased risk of infection, bruising or bleeding
- feeling weak or having no energy
- tiredness (fatigue)
- diarrhoea
- feeling or being sick
- tummy (abdominal pain)
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Sanjay Popat
Supported by
European Thoracic Oncology Platform (ETOP)
F. Hoffmann - La Roche, Limited
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040