A trial looking at ibrutinib and standard treatment for children and young adults with B cell non-Hodgkin lymphoma (SPARKLE)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is for children and young people whose lymphoma has come back or treatment has stopped working.
It is a trial in children and young adults up to the age of 30 who were diagnosed with B cell non-Hodgkin lymphoma under the age of 18.
We use the term ‘you’ in this summary. But if you are a parent, we are referring to your child.
More about this trial
- RICE
- RVICI
- rituximab
- vincristine
- ifosfamide
- carboplatin
- idarubicin
- rituximab
- ifosfamide
- carboplatin
- etoposide
- how safe treatment is
- if adding ibrutinib to RICE or RVICI improves treatment
- more about the side effects
Who can enter
- have B cell non-Hodgkin lymphoma (NHL) such as Burkitt lymphoma, diffuse large B cell lymphoma (DBCL) or other childhood B cell lymphomas
- have had your first treatment but the lymphoma has come back, or the standard treatments have stopped working
- have an area of lymphoma that measures at least 1cm across on a scan, you have bone marrow involvement or you have lymphoma in the spinal fluid (CSF)
- have satisfactory blood test results
- need quite a lot of help to care for yourself (Karnofsky performance scale 50 or more) or you get dressed but need to lie down for much of the day and only take part in quiet play and activities (Lansky play scale 50 or more)
- are willing to use reliable contraception during treatment and for 3 months after the last dose of ibrutinib if you are sexually active and there is any chance you or your partner could become pregnant or for at least 1 year after the last dose of RICE or RVICI
- are between 1 years old and 30 to join part 2 if you were diagnosed with NHL under the age of 18
- have had ibrutinib in the past
- have had a stem cell transplant in the last 6 months
- developed lymphoma after having a stem cell transplant or an organ transplant (this is called post transplant lymphoproliferative disease or PTLD)
- have had an experimental treatment or device within 30 days of starting trial treatment
- are having treatment to thin the blood such as warfarin
- have a problem with how your blood clots
- you take medication that blocks a substance called CYP3A4/5
- have problems with your heart, such as an abnormal heart rhythm or it is weak
- have a problem with your digestive system that means you can’t absorb ibrutinib properly
- have had major surgery within 4 weeks of joining the trial, have surgery planned during the trial or you haven’t fully recovered from an operation
- have HIV
- have an active hepatitis B or hepatitis C infection
- have any other medical condition or mental health problem that the trial team think would affect you taking part
Other
You:
- are pregnant or breastfeeding or planning a pregnancy within 3 months of stopping ibrutinib or within a year of stopping the other drugs in the trial
- are allergic to ibrutinib, any of the other drugs in the trial or anything they contain
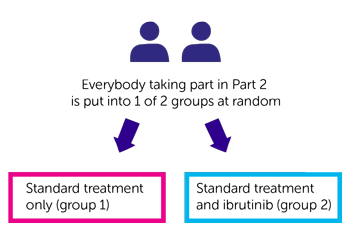
Trial design
- standard treatment only (group 1)
- standard treatment and ibrutinib
(group 2)
- see how well the treatment is working
- find out what happens to the drugs in the body
- look for biomarkers to predict who will benefit from treatment
Hospital visits
- physical examination
- blood samples
- urine samples
- heart trace (ECG)
- heart scan (echocardiogram or MUGA scan)
- CT scan, MRI scan or PET scan
- lumbar puncture to collect a sample of fluid that circulates around the brain and spinal cord
- bone marrow test
- every 3 months in the first year
- every 6 months in the second and third year
Side effects
- diarrhoea or constipation
- muscle pain, bone pain and joint aches
- feeling or being sick
- an increased risk of infection
- skin rash or skin infections
- an increased risk of bruising and bleeding
- high temperatures
- colds
- pneumonia
- swelling of the hands or feet
- muscle spasms
- sores in the mouth
- headache
- high blood pressure
- sinus infection
- rituximab
- vincristine
- ifosfamide
- carboplatin
- idarubicin
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Prof Amos Burke
Supported by
Janssen-Cilag Limited
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040