A trial looking at lenvatinib for people with neuroendocrine tumours that have spread (TALENT)
Cancer type:
Status:
Phase:
This trial was for people with a neuroendocrine tumour that had started in the pancreas, stomach or bowel.
This trial was open for people to join between October 2016 and September 2017.
More about this trial
The treatment for NET is often surgery. But sometimes the cancer can spread to other parts of body. This is metastatic cancer.
Doctors wanted new ways to help these people. In this trial, they looked at a drug called lenvatinib.
Lenvatinib is a targeted cancer drug. It works by blocking certain proteins that help cells to grow blood vessels. All cancer cells need blood vessels to survive and grow. Doctors thought lenvatinib could stop the tumour growing.
This trial had 2 groups. People in one group had a NET that started in the pancreas. People in the other group had a NET that started in the stomach or bowel.
- find out how well lenvatinib worked
- learn more about the side effects
- look for substances (biomarkers) that could help tell how well treatment worked
Summary of results
The team found that lenvatinib worked well for people with neuroendocrine tumours that started in the pancreas, stomach or bowel that had spread.
About this trial
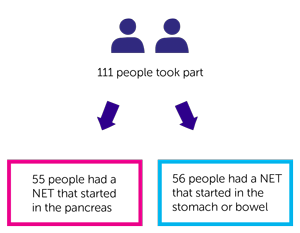
This was a phase 2 trial. 111 people took part:
- 55 people had a NET that started in the pancreas (cohort A)
- 56 people had a NET that started in the stomach or bowel (cohort B)
Everyone had a scan of where their tumour had spread:
- before starting treatment
- during treatment
- when they finished treatment
Researchers compared these scans to see how well lenvatinib worked.
Results
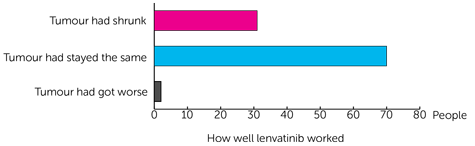
Of the 111 people the team had results for 106 people. They found that for:
- 31 people (29.2%) their tumour had shrunk (a partial response)
- 70 people (66%) their tumour had stayed the same (stable disease)
- 2 people (2%) their tumour had got worse (progressive disease)
- 21 people (40.4%) their tumour had shrunk
- 29 people (55.8%) their tumour had stayed the same
- 2 people (3.8%) their tumour had got worse
Of the 56 people with a NET that started in the stomach or bowel researchers had the results of 54 people. They found that for:
- 10 people (18.5%) their tumour had shrunk
- 41 people (76%) their tumour had stayed the same
3 people (2.8%) from this group didn’t have scans. So the team weren’t able to assess how well their treatment worked.
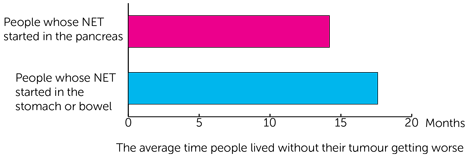
The team also looked at the average time people lived without their tumour getting worse. They found it was:
- just over 14 months (14.2) for people whose NET started in the pancreas
- just over 17½ months (17.6) for people whose NET started in the stomach or bowel
Side effects
The team found that the side effects were the same as reported in other trials with lenvatinib. These included:
- feeling or being sick
- weakness and tiredness (fatigue)
- high blood pressure
- diarrhoea
- loss of appetite
- headache
Samples
Researchers are still looking at the samples they collected. When results for these are available this summary will be updated.
Conclusion
The trial team concluded that lenvatinib worked well for people with a NET that had started in the pancreas, stomach or bowel that had spread.
Lenvatinib showed promise in stopping NETs coming back for people who had already had treatment.
Where this information comes from
We have based this summary on information from the research team. The information they sent us has been reviewed by independent specialists (peer reviewed) but may not have been published in a medical journal. The figures we quote above were provided by the research team. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Juan Valle
Supported by
Grupo Español de Tumores Neuroendrocrinos (GETNE)
The Christie NHS Foundation Trust
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040