A trial looking at using ultrasound and biomarkers in treatment for pleural effusion (SIMPLE)
Cancer type:
Status:
Phase:
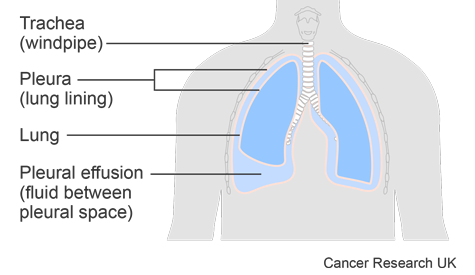
This trial was for people who had a pleural effusion caused by cancer. A pleural effusion is a build up of fluid in the space between the sheets of tissue that cover the outside of the lung and the lining of the chest cavity. These sheets of tissue are called the pleura.
The trial was open for people to join between 2016 and 2019. The team published the results about ultrasound scans in 2022. And the results about  in 2018.
in 2018.
More about this trial
A pleural effusion can cause:
- shortness of breath
- an aching chest
- discomfort and heaviness
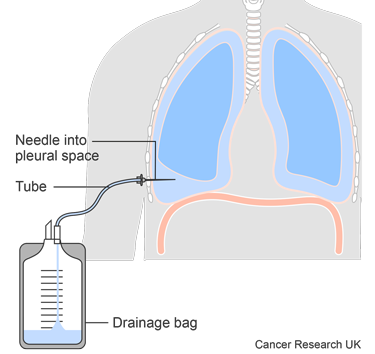
You might have a chest tube to remove the fluid as a treatment for pleural effusion.
Once the fluid stops draining, your doctor removes the tube.
One possible treatment to stop fluid from building up and help relieve symptoms is called pleurodesis. It seals the space between the tissues covering the lung by using sterile talc to make them inflamed so they stick together. Then there is no space for the fluid to collect.
The treatment works well for most people, but not for everyone. In this trial doctors wanted to see if there was a way that they could improve pleurodesis treatment.
A smaller study had shown that using regular ultrasound scans could show if pleurodesis was likely to work quite early on. This was by looking for a certain movement of the lungs inside the chest. Less movement showed that pleurodesis was working. The researchers wanted to look at this further.
They compared the ultrasound scans to . This is when doctors looked at how much fluid was being drained by a chest tube as a sign that the treatment was working.
. This is when doctors looked at how much fluid was being drained by a chest tube as a sign that the treatment was working.
The main aims of the trial were to:
- see if using daily ultrasound scans could show how well the treatment was working compared to standard care
- find out if using ultrasound scans meant that people spent less time in hospital than those having standard care
- test any fluid they drained from the pleura to see if there were any
biomarkers  that could tell them how well the treatment would work
that could tell them how well the treatment would work
Summary of results
This trial showed that people having an ultrasound scan spent, on average, a day less in hospital than with the standard care.
After 3 months the researchers found that the ultrasound guided treatment worked as well as having standard care.
The researchers did not find any biomarkers from the fluid samples to show how well pleurodesis would work.
The fluid sample results helped the researchers to develop a score to predict how long people are likely to live.
Results for ultrasound scans
This was a randomised trial in the UK and the Netherlands. 313 people took part. Everyone had pleurodesis and:
- 159 people had ultrasound scans
- 154 people had standard care
Pleurodesis didn’t work for everyone. The team were able to look at how well the treatment worked after 3 months in:
- 91 people in the ultrasound scan group
- 109 people in the standard care group
They found that the two ways of doing pleurodesis worked as well as each other. At 3 months it was successful in:
- 64 out of 91 people (70%) in the ultrasound scan group
- 75 out of 109 people (69%) in the standard care group
The trial team looked at how long people from each group stayed in hospital around the time of having pleurodesis.
It was on average:
- 2 days for people in the ultrasound scan group
- 3 days for people in the standard care group
The researchers say the costs to the NHS were similar for each group. But that ultrasound scans were good value when compared to standard care.
The researchers did not look at the side effects of the two types of treatment.
They asked the people taking part in the trial about how their symptoms and  around the time of the trial treatment. There were no important differences between the two groups.
around the time of the trial treatment. There were no important differences between the two groups.
Results for biomarker samples
The researchers looked at the samples along with samples from other research studies.
This included 162 samples from either:
- people taking part in the SIMPLE trial
- stored samples from other people with pleural effusion from cancer. These were from a biobank in Oxford. Biobanking is storing samples from an operation or tests for research.
The researchers used the samples to test a new way of predicting how long people would live. This is called the PROMISE score and uses different factors including:
- cancer type
- type of treatment
- blood tests results
- amount of protein in the fluid sample
The researchers found the PROMISE score worked well. They say it could be used by doctors to help them with treatment options.
Conclusion
This trial shows that using ultrasounds for pleurodesis can reduce time in hospital without changing how likely it is to work.
The researchers say that these results may support making ultrasounds part of standard care for this type of pleurodesis.
The biomarker results helped researchers show that the PROMISE score is an accurate way of predicting how long someone may live with pleural effusion caused by cancer.
Biomarkers did not help predict how well pleurodesis would work.
More detailed information
There is more information about this research in the references below.
Please note, the information we link to here is not in plain English. It has been written for healthcare professionals and researchers.
Role of thoracic ultrasonography in pleurodesis pathways for malignant pleural effusions (SIMPLE): an open-label, randomised controlled trial
I Psallidas and others
The Lancet Respiratory Medicine, 2022. Volume 10, issue 2, pages 139-148.
Development and validation of response markers to predict survival and pleurodesis success in patients with malignant pleural effusion (PROMISE): a multicohort analysis
I Psallidas and others
The Lancet Oncology, 2018. Volume 19, issue 7, pages 930-939.
Where this information comes from
We have based this summary on the information in the articles above. These have been reviewed by independent specialists ( ) and published in medical journals. We have not analysed the data ourselves.
) and published in medical journals. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Najib Rahman
Supported by
European Respiratory Society
Marie Curie Cancer Care
NIHR Clinical Research Network: Cancer
Slater & Gordon Research Fund
University of Oxford
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040