A trial looking at reducing further operations after breast conserving surgery
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is looking at whether a new device called a MarginProbe can reduce the number of operations women need after breast conserving surgery. The MarginProbe measures the edges (the margins) of any tissue removed during surgery to make sure they are clear of cancer cells.
More about this trial
In breast conserving surgery the surgeon takes away just the cancer and a border of healthy tissue all around it. They leave behind as much healthy breast tissue as possible.
The surgeon uses various ways to make sure that they have removed the cancer. As well as checking and feeling the breast tissue they can use scans.
Your surgeon sends the breast tissue they have removed to a pathologist for examination under a microscope. The pathologist checks for cancer cells in the margins around the tissue. You may need more surgery if there was no clear margin of tissue around the lump or area of cancer.
In this trial doctors are going to compare 2 groups. One group has the standard breast conserving surgery. In the other group the surgeon will use the MarginProbe.
The aim of the trial is to see if the MarginProbe reduces the amount of surgery women need to have after breast conserving surgery.
Who can enter
The following bullet points list the entry conditions for this trial. Talk to your doctor or the trial team if you are unsure about any of these. They will be able to advise you.
You may be able to join this trial if you are a woman in one of the following situations. You have:
- A type of breast cancer called invasive ductal cancer with surrounding ductal carcinoma in situ (DCIS)
- An invasive lobular cancer which is larger than 1.5 cm
- HER 2 positive breast cancer
And all of the following must apply. You are:
- Able to have breast conserving surgery
- Aged between 18 and 90
You cannot join this trial if any of these apply. You
- Have either a large breast cancer (bigger than 4 cm) or a small invasive breast cancer (smaller than 1.5 cm)
- Have cancer in 2 separate areas of your breast
- Have cancer in both breasts
- Have an implant in the breast which is due to be operated on
- Are not able to have radiotherapy
- Have had radiotherapy to the breast and you are due to have surgery to it
- Are due to have an anti cancer treatment that reaches your whole body (systemic therapy) before your surgery
- Are due to have a procedure called cryo assisted localisation
- Are pregnant or breastfeeding
Trial design
The researchers need around 470 women to take part in this trial.
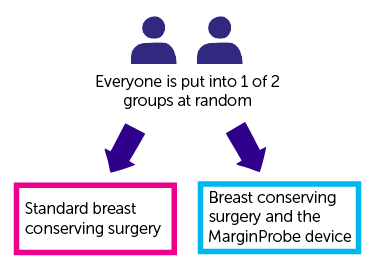
It is a randomised trial. You are put into 1 of 2 treatment groups by computer. Neither you nor your doctor can choose which group you are in.
You will be put into one of the treatment groups after you are in theatre for your surgery. You will not be told which group you are in.
- One group has standard breast conserving surgery
- The other group has breast conserving surgery and the MarginProbe.
Most women have their surgery as a day case.
Quality of life
You’ll be asked to complete some questionnaires
- before surgery
- 1, 3 and 9 months after your surgery
These will ask questions about your quality of life and how your breast looks (cosmetic outcomes).
Photographic measurements
The researchers also need to take some photographic measurements of your breast before your surgery, if you haven’t already had them taken.
You’ll also have these done again 9 months after your surgery.
Hospital visits
You go to hospital to have your surgery.
2 weeks after your surgery you have an appointment. At this appointment the surgeon will let you know if the breast tissue margins were clear and whether you need to have any further surgery.
As part of your routine care you will have regular check ups with your doctor. They will explain how often they need to see you.
Side effects
Women in the group using the MarginProbe may have extra breast tissue removed. But they may not need to go on to have any extra surgery.
We have information about surgery for breast cancer.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Nigel Bundred
Supported by
NIHR: Research for Patient Benefit
University Hospital of South Manchester NHS Trust
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



