A trial looking at MEDI4736 and tremelimumab for people with head and neck cancer (KESTREL)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is for people with head and neck cancer that has either come back (recurrent cancer) or spread elsewhere (metastatic cancer). It is looking at MEDI4736 alone or MEDI4736 and tremelimumab compared to standard treatment
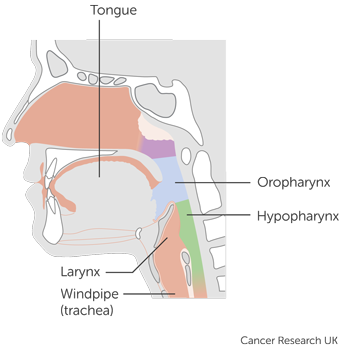
It is for people with head and neck  of the
of the
More about this trial
Doctors usually treat recurrent or metastatic head and neck cancer using a combination of 3 different chemotherapy drugs
- cetuximab
- 5-fluorouracil
- carboplatin or cisplatin
This is the  . You may hear this treatment called the EXTREME regimen.
. You may hear this treatment called the EXTREME regimen.
MEDI4736 and tremelimumab are types of biological therapies called monoclonal antibodies. They seek out cancer cells by looking for a particular protein and attaching to it. MEDI4736 and tremelimumab seek out 2 different proteins.
Researchers think they may help your immune system attack your cancer and stop it from growing.
In this trial, people will have 1 of the following
- MEDI4736
- MEDI4736 and tremelimumab
- Standard treatment
The aims of this trial are to
- find out how well MEDI4736 and tremelimumab work as a treatment
- find out what happens to MEDI4736 and tremelimumab in your body
- learn more about side effects
- learn how well people cope with side effects
Who can enter
The following bullet points list the entry conditions for this trial. Talk to your doctor or the trial team if you are unsure about any of these. They will be able to advise you.
You may be able to join this trial if all of the following apply
- You have squamous cell carcinoma of the head and neck that has come back or spread to another part of your body
- You are not able to have any treatment to cure your cancer such as surgery or radiotherapy
- You are willing to have a sample of your cancer taken (a
biopsy  ) if there is no suitable sample available that is less than 3 years old
) if there is no suitable sample available that is less than 3 years old - You have satisfactory blood tests results
- You have a satisfactory heart rate
- You have at least 1 area of cancer that hasn’t been treated with
radiotherapy  , can be seen on a scan and measures at least 10 mm
, can be seen on a scan and measures at least 10 mm - You are well enough to carry out your normal activities, apart from heavy physical work (performance status of 0 or 1)
- You are at least 18 years old
- You are willing to use reliable contraception during treatment and for 3 months after the final dose of MEDI4736 or for 6 months after the last dose of MEDI4736 and tremelimumab if there is any possibility you or your partner could become pregnant. If you have standard treatment, the trial team will advise you how long you should use reliable contraception for
You cannot join this trial if any of these apply
- Your cancer has spread to your brain, the tissues surrounding your brain or your
spinal cord 
- You have had another cancer in the last 5 years apart from cancers that have not spread (
carcinoma in situ  ) and that have been successfully treated
) and that have been successfully treated - You have had treatment that reached your whole body (
systemic treatment  ) after your cancer came back or spread
) after your cancer came back or spread - Your head and neck cancer has returned or spread within 6 months of the last dose of
platinum chemotherapy 
- You have had radiotherapy in the last 30 days
- You have had chemotherapy or a biological therapy in the last 21 days
- You have had a new experimental treatment in the last 28 days
- You have had major surgery in the past 28 days (if you have had a small operation to remove some of your cancer and improve your symptoms you may still be able to take part)
- You have moderate to severe side effects from previous anti cancer treatments (apart from hair loss, changes to your skin called vitiligo and lower than normal levels of a type of white blood cell called a lymphocyte)
- You have taken drugs that damp down your immune system (immunosuppressants) such as steroids in the past 2 weeks
- You have had an organ transplant
- You have had an
autoimmune disease  in the past 3 years
in the past 3 years - You have problems with your
digestive system  such as colitis, Crohn’s disease or diverticulitis
such as colitis, Crohn’s disease or diverticulitis - You have heart problems such as congestive heart failure, high blood pressure that is not controlled by medication or angina that is not controlled
- You have an active
infection 
- You have HIV
- You have hepatitis B or hepatitis C
- You have had tuberculosis (TB)
- You weigh less than 30 kg (4 stone 10lb)
- You are allergic to MEDI4736, tremelimumab or anything it contains
- You have had a live
vaccination  in the past 30 days
in the past 30 days - You have any other serious medical condition or mental health problem that the trial team think could affect you taking part
- You are pregnant or breast feeding
Trial design
This is an international phase 3 trial. The researchers need about 628 people to take part worldwide and hope that around 17 people from the UK will take part.
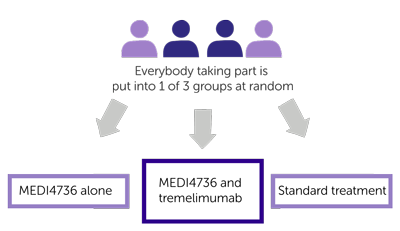
This trial is randomised. The people taking part are put into 1 of the following treatment groups by computer
- MEDI4736 alone
- MEDI4736 and tremelimumab
- Standard treatment
Neither you nor your doctor will be able to decide which group you are.
You are 2 times more likely to have MEDI4736 and tremelimumab than MEDI4736 alone or standard treatment.
MEDI4736 alone
You have MEDI4736 as a drip into a vein every 4 weeks. It takes about 1 hour.
You continue to have MEDI4736 for as long as the doctor feels it is helping you, even if your cancer gets worse.
MEDI4736 and tremelimumab
You have MEDI4736 and tremelimumab, as a drip into a vein every 4 weeks. Each drug takes about 1 hour. The first time you have the drugs there will be a 1 hour gap between MEDI4736 and tremelimumab.
You have both drugs for 4 months. After that you have MEDI4736 alone for as long as the study doctor feels it is helping you and the side effects aren’t too bad.
Standard treatment (EXTREME)
You have a combination of 3 drugs
- cetuximab
- 5-fluouracil
- cisplatin or carboplatin (your doctor will explain which drug is best for you)
You have all 3 drugs through a drip into a vein. The trial team can give you more information on how often you have them.
Quality of life
Everybody taking part of this trial will be asked to complete a quality of life questionnaire before starting treatment, at set times during the trial and then after you finish your treatment.
It will ask about how you have been feeling and what side affects you have had.
Blood tests
You have some extra blood tests as part of this trial. The researchers want to find out what happens to MEDI4736 and tremelimumab in the body.
They will also look for substances called  to see why the treatments work better for some people than others.
to see why the treatments work better for some people than others.
Tissue samples
You may also need to give a tissue sample ( ) of your cancer before you start treatment. This is to check if there is a marker on your cancer called PD-L1.
) of your cancer before you start treatment. This is to check if there is a marker on your cancer called PD-L1.
If you have oropharyngeal cancer they will also check if there is a virus present on your cancer called human papilloma virus (HPV). If you already had this test done in the past, the trial team will check the result before you start treatment.
Hospital visits
You see a doctor and have some tests before taking part. These tests include:
If you have MEDI4736 alone or MEDI4736 and tremelimumab you see your doctor for blood tests and a physical examination every 4 weeks.
If you have standard treatment you see the doctor for blood tests and a physical examination every 3 weeks.
You have a CT or a MRI scan every 6 weeks while you are having treatment. After 6 months you have a CT or a MRI scan every 8 weeks.
When you finish your treatment you see the trial team
- once a month for the first 3 months
- every 2 months
You have a scan every 2 months. This will continue for as long as your cancer stays the same and does not get worse. If your cancer gets worse the trial team will phone you every 3 months to see how you are.
Side effects
MEDI4736 and tremelimumab are new drugs and there may be side effects we don’t know about yet.
The trial team monitor you during the time you have treatment and you will be given a phone number to call them if you are worried about anything. The team will tell you about all the possible side effects before you start the trial.
The most common side effects of MEDI4736 and tremelimumab are
- diarrhoea
- skin rashes
- liver problems such as inflammation of the liver (hepatitis) and a high level of liver enzymes on your blood
- feeling or being sick
- loss of appetite
We have information on
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Hisham Mehanna
Supported by
AstraZeneca
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



