A trial looking at cobimetinib and paclitaxel for triple negative breast cancer
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is looking at a new drug called cobimetinib and a chemotherapy drug called paclitaxel for triple negative breast cancer. This means the breast cancer doesn’t have receptors for the hormones progesterone and oestrogen, or for the protein HER2. The trial is for people whose cancer has grown into surrounding tissue (is locally advanced) or has spread to another part of their body.
More about this trial
Doctors sometimes use a chemotherapy drug called paclitaxel for triple negative breast cancer. This works well but doctors are looking at ways to improve treatment. In this trial, they are looking at a drug called cobimetinib.
Cobimetinib is a type of biological therapy. It works by targeting a protein called MEK and stop signals that cancers use to divide and grow. The researchers think that combining cobimetinib with paclitaxel may improve treatment.
This trial will compare cobimetinib and paclitaxel with paclitaxel alone. The aims of the trial are to
- Find out if the combination is safe to give
- Find out if having cobimetinib and paclitaxel helps people who have triple negative breast cancer that has spread locally or to another part of their body
- Learn more about the side effects and how this affects quality of life
Who can enter
The following bullet points list the entry conditions for this trial. Talk to your doctor or the trial team if you are unsure about any of these. They will be able to advise you.
You may be able to join this trial if all of the following apply
- You have breast cancer that has grown into tissue surrounding your breast and it can’t be removed with surgery, or it has spread to another part of your body
- Your breast cancer doesn’t have receptors for oestrogen (oestrogen receptor positive) or progesterone or for the HER2 protein (triple negative breast cancer)
- Your cancer can be measured on a scan
- You are well enough to take carry out your normal activities, apart from heavy physical work (performance status 0 or 1)
- You have satisfactory blood test results
- You are willing to use reliable contraception during the trial and for 6 months afterwards, if there is any chance that you or your partner could become pregnant
- You are at least 18 years old
You cannot join this trial if any of these apply. You
- Have cancer that has spread to your brain or spinal cord and is getting worse or is causing symptoms
- Have had hormone therapy or biological therapy for locally advanced or breast cancer that has spread
- Have had had chemotherapy for breast cancer spread (you may still be able to take part if you had chemotherapy before or after your breast cancer surgery and this was more than 6 months ago)
- Have had any other type of treatment for your cancer in the last 3 weeks or you have had radiotherapy for cancer spread in the last month
- Had treatment when you were first diagnosed with breast cancer that included drugs called bevacizumab or sorafenib, or types of drugs that block blood vessel growth
- Have had drugs called PI3K inhibitors, AKT inhibitors, or mTOR inhibitors
- Have had experimental treatment with drugs called Raf, MEK or MAPK
- Are taking St John’s wort (you may still take part in the trial if you stop taking it a week before you join but you should discuss this with your doctor first)
- Have had trastuzumab (Herceptin)
- Have certain eye conditions
- Have nerve damage affecting your hands or feet (peripheral neuropathy) unless it is quite mild
- Have had major surgery in the last month
- Have an infection and need to have antibiotics through a drip into a vein
- Have certain heart problems
- Are known to be very sensitive to paclitaxel
- Have any other medical condition or mental health problem that the trial team thinks would affect you taking part in this trial
- Are pregnant or breastfeeding
Trial design
This is a phase 2 trial. The researchers need 112 people to join.
This study is in 2 parts. In part 1, the researchers are looking at the side effects of having cobimetinib and paclitaxel. In the 2nd part, the researchers want to compare cobimetinib and paclitaxel with paclitaxel alone.
In part 1, 12 patients will have cobimetinib alongside paclitaxel. When the researchers have know more about the side effects then part 2 can begin.
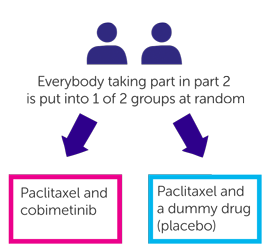
Part 2 is randomised. 100 people will join this part of the trial. The people taking part are put into 2 different treatment groups by a computer. Neither you nor your doctor will be able to decide which group you are in.
- One group have paclitaxel and cobimetinib
- The other group have paclitaxel and a dummy drug (placebo)
You have paclitaxel through a drip into a vein. You have it once a week for 3 weeks out of every 4. This takes about an hour each time. Each 4 week period is called a cycle of treatment.
Cobimetinib is a tablet. You have either cobimetinib or dummy tablets (which look exactly the same). You take 2 or 3 tablets once a day for 3 weeks of each treatment cycle starting on the 3rd day of the treatment cycle.
You have treatment for as long as it is helping you and the side effects aren’t too bad.
During the trial, the researchers take a number of blood samples. They will also ask for a sample of your cancer that was removed when you had surgery or a biopsy in the past and for another biopsy if your cancer gets worse. The researchers will use these samples to look for substances called biomarkers that may help them understand more about how cobimetinib and paclitaxel work together and how the drugs affect your tumour.
The trial team will ask everybody taking part in the trial to complete some questionnaires before treatment starts and then regularly throughout treatment. These are called quality of life questionnaires. They look at how treatment affects you physically and emotionally.
Hospital visits
You see the trial doctor to have some tests before taking part in the trial. These include
- Physical examination
- Blood and urine tests
- Heart trace (ECG) or MUGA
- CT scan or MRI scan
- Bone scan
- Eye examination
You go to hospital once a week for 3 weeks to have the paclitaxel. You take the cobimetinib or dummy drug at home. You see the trial doctor twice a month for a physical examination. You have regular blood tests and eye tests during treatment.
You have a CT or MRI scan every 2 months until your cancer gets worse. When you finish treatment the trial team will contact you every 3 months. They will either phone you or check your medical records to see how you are.
Side effects
As cobimetinib is a new drug, there may be side effects that we don’t know about yet. The most common side effects so far include
- A drop in red blood cells causing tiredness
- Diarrhoea or constipation
- Feeling or being sick
- Tummy pain
- Swelling in your face, arms or legs
- Tiredness (fatigue)
- Changes to your liver
- Loss of appetite
- Low levels of potassium in your blood
- Dizziness
- Skin rash
There is a very small risk of developing blurred vision while having cobimetinib. This is caused by a build up of fluid within the layers around the eye lens (the retina). This side effect is usually temporary and goes away when you stop taking the drug. You will have regular eye examinations during the trial to check for this.
We have information about the side effects of paclitaxel.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Tamas Hickish
Supported by
Roche
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



