A trial of pixantrone for non Hodgkin lymphoma (PIX-R)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is looking at a chemotherapy drug called pixantrone for non Hodgkin lymphoma. The trial is for people with a type of non Hodgkin lymphoma called diffuse large B cell lymphoma (DLBCL) or follicular lymphoma.
More about this trial
Doctors can treat DLBCL and follicular lymphoma with  , such as mitoxantrone or
, such as mitoxantrone or  , in combination with a biological therapy called rituximab.
, in combination with a biological therapy called rituximab.
If the lymphoma comes back after this, they may suggest a stem cell transplant. Stem cell transplant is a very intensive treatment. So some people can’t have a transplant or choose not to. The researchers think that pixantrone may be able to help these people.
In this trial researchers will compare the combination of pixantrone and rituximab with gemcitabine and rituximab.
Pixantrone is a chemotherapy drug that works in a similar way to mitoxantrone and anthracycline chemotherapy drugs, such as doxorubicin.
Gemcitabine is another chemotherapy drug. We know from research that the combination of gemcitabine and rituximab can benefit people with non Hodgkin lymphoma that has come back and who can’t have a stem cell transplant.
Rituximab is a biological therapy called a monoclonal antibody. These seek out cancer cells by looking for a particular protein.
The main aim of this trial is to find how well the combination of pixantrone and rituximab works for people with DLBCL or follicular lymphoma.
Who can enter
You may be able to join this trial if all of the following apply
- You have a type of non Hodgkin lymphoma called diffuse large B cell lymphoma (DLBCL) or follicular lymphoma that is fast growing (grade 3)
- You are not able to, or don’t choose to, have
high dose chemotherapy  followed by a
followed by a stem cell transplant 
- You have already had chemotherapy for your lymphoma. How much chemotherapy depends on which type of lymphoma you have, if it was newly diagnosed or had transformed from low grade to high grade. The trial doctor can advise you about this
- You have had rituximab as part of your treatment
- Your treatment finished at least 28 days ago
- You have at least one area of lymphoma that can be measured and hasn’t been treated with
radiotherapy  . If it is a
. If it is a lymph node  it must be at least 1½ cm in diameter. If it isn’t a lymph node, the area of lymphoma must be more than 1 cm in diameter
it must be at least 1½ cm in diameter. If it isn’t a lymph node, the area of lymphoma must be more than 1 cm in diameter - You are well enough to be up and about for at least half the day (performance status 0, 1 or 2)
- You have satisfactory blood test results
- Your heart works well enough (the trial team will test you for this)
- You are willing to use reliable contraception during treatment and for at least a year afterwards if there is any chance you or your partner could become pregnant
- You are at least 18 years old
You cannot join this trial if any of these apply
- You have lymphoma in the brain or spinal cord. You may be able to join if it was in one area only, hasn’t come back for more than a year and cannot be seen on an
MRI scan 
- You only have lymphoma in lymph nodes that cannot be seen on a scan, or on your skin or in your
bone marrow 
- Your lymphoma had got worse within 12 weeks after completing chemotherapy
- You have had a certain amount of a chemotherapy drug called
doxorubicin  (your doctor can advise you about this)
(your doctor can advise you about this) - You have any ongoing serious side effects from treatment apart from hair loss
- You have had major surgery in the month before being put into a treatment group for this trial
- You have had another cancer in the past 5 years apart from
non melanoma skin cancer  and some
and some early cancers 
- You have certain heart problems (the trial team can advise you about this)
- You have HIV, hepatitis B or hepatitis C
- You have any other medical or mental health problem that the trial team think would affect you taking part
- You are taking certain medications (the trial team can advise you about this)
- You have had an experimental drug as part of another clinical trial in the past month before being put into a treatment group in this trial
- You are allergic to the drugs used in this trial
- You are pregnant or breast feeding
Trial design
This is an international phase 3 trial. The researchers need about 320 people to join.
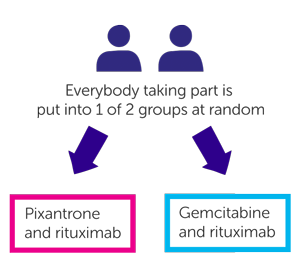
It is a randomised trial. The people taking part are put into treatment groups by a computer. Neither you nor your doctor will be able to decide which group you are in.
- People in one group have pixantrone and rituximab
- People in the other group have gemcitabine and rituximab
You have all the drugs as a drip into a vein in your arm. You have pixantrone or gemcitabine once a week for 3 weeks then have a week of no treatment. You have rituximab once every 4 weeks. Each 4 week period is called a cycle of treatment.
As long as it is helping and the side effects aren’t too bad you can have up to 6 cycles of treatment.
Hospital visits
You see the doctor to have some tests before taking part. These tests include
- A physical examination
- Blood tests
- Urine test
- CT scan
- Heart trace (
ECG  )
) - Heart scan (
ECHO  )
)
When you have rituximab you will need to stay at the hospital for at least 2 hours afterwards. This is to make sure you don’t have a reaction to it.
During treatment you see the doctor every week for the first 3 weeks, then once a week for a physical examination and blood tests. You have another heart scan before cycle 3 and cycle 5 of your treatment. You have a CT scan every 8 weeks.
A month after finishing treatment you see the doctor for
- A physical examination
- Blood tests
- PET scan
- Bone marrow test
- Heart trace
- Heart scan
You then see the doctor every 8 weeks for 6 months and then every 12 weeks for 18 months.
If you stopped treatment because of side effects or your lymphoma got worse, you see the doctor every 3 months.
Side effects
The most common side effects of pixantrone include
- A drop in blood cells causing an increased risk of infection, bruising or bleeding
- Changes to the way your heart, liver and kidneys work
- High temperature (fever)
- Weakness
- Dizziness
- Tiredness (fatigue)
- Feeling or being sick
- Constipation
- Taste changes and loss of appetite
- General pain
- Hair loss
- Cough
- Rash, skin discolouration
- Changes to the colour of your urine
- Ringing in the ears (tinitus)
- Developing another cancer such as myelodysplastic syndrome (MDS) or acute myeloid luekaemia (AML)
The trial doctor will talk to you about the possible side effects of all the drugs used in this trial before you agree to take part.
We have more information about the side effects of gemcitabine and rituximab.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Ruth Pettengell
Supported by
CTI BioPharma Corp
Experimental Cancer Medicine Centre (ECMC)
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040