A trial of pembrolizumab and chemotherapy for cancer of the stomach and cancer where the food pipe joins the stomach (KEYNOTE 859)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
- has grown into the surrounding tissues (locally advanced) or spread to other parts of the body (advanced)
- doesn’t have a large amount of proteins called HER2 on its surface (HER2 negative)
More about this trial
- cisplatin and fluorouracil (5FU)
- capecitabine and oxaliplatin
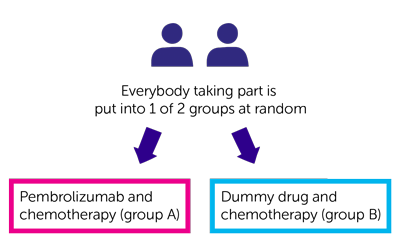
- pembrolizumab and chemotherapy (group A)
- dummy drug (placebo) and chemotherapy (group B)
- find out whether pembrolizumab helps people with stomach and gastro oesophageal junction cancer
- learn more about the side effects
Who can enter
- you have stomach cancer or gastro oesophageal junction cancer
- your cancer has grown into the surrounding tissues (locally advanced) and can’t be removed with surgery or it has spread to other parts of the body (advanced cancer)
- your cancer does not have a large amount of HER2 protein on its surface (HER2 negative)
- you have at least an area of cancer that doctors can see and measure on a scan
- you are willing to give a new sample of tissue (biopsy) if there isn’t a suitable sample available that doctors can test for certain proteins and biomarkers
- your cancer is either PD-L1 positive or PD-L1 negative (the trial team will test a sample of tissue for this)
- you are able to carry out your normal activities apart from heavy physical work (performance status of 0 or 1)
- you have satisfactory blood test results
- you are willing to use reliable contraception during treatment and for up to 6 months afterwards if there is any possibility that you or your partner could become pregnant
- you are at least 18 years old
- have a rare type of stomach cancer called squamous cell or undifferentiated stomach cancer
- have cancer spread in your brain, spinal cord or the membranes that surround your brain (carcinomatous meningitis) unless you have had treatment for it, you have stopped taking steroids 2 weeks ago and a scan showed that it has been stable for the past month
- have had treatment for locally advanced or advanced cancer. You might be able to take part if it was chemotherapy before or after surgery
- have had pembrolizumab or any other similar drug
- have had a cancer treatment that reached your whole body (systemic treatment) in the last month
- have moderate or severe side effects from previous treatment
- have had radiotherapy in the past 2 weeks and you still have side effects from it (1 week if it was radiotherapy to help with symptoms, apart from radiotherapy to the brain or the spinal cord)
- have had another cancer in the past 5 years apart from non melanoma skin cancer or carcinoma in situ of the breast and cervix that has been successfully treated
- are taking part, or have taken part in another clinical trial using an experimental drug or device in the past month
- have an autoimmune disease that needed treatment that reached your whole body in the past 2 years, unless it was treatment to replace something that the body makes such as thyroxine or insulin
- have taken drugs that damp down your immune system (steroids) in the past week, unless it was a very small dose, a cream or inhaler
- have had a major surgery in the past month or you might need one during this trial
- still have side effects from previous surgery
- have, or have had a lung inflammation (pneumonitis) that needed treatment with steroids
- have had an organ or tissue transplant from a donor
- have an infection that needs treatment
- have HIV
- have hepatitis B or hepatitis C
- have tuberculosis (TB)
- have moderate or severe hearing loss if you are going to have treatment with cisplatin
- have moderate or severe numbness or tingling in fingers and toes
- have any other medical condition or mental health problem that the trial team think could affect you taking part
- are pregnant or breastfeeding
- have had a live vaccine in the past month
- are sensitive to pembrolizumab, any of the drugs used in this trial or anything they contain
Trial design
- pembrolizumab and chemotherapy (group A)
- dummy drug and chemotherapy (group B)
- completing 2 years of treatment
- you stopped treatment because there was no sign of your cancer
- between 1 and 2 hours to have cisplatin
- about 5 days to have 5FU
- cisplatin on the 1st day of each cycle
- 5FU continuously over 5 days starting on the 1st day of each cycle
- oxaliplatin as a drip into your vein every 3 weeks, taking around 2 hours each time you have it
- capecitabine as tablets that you swallow whole twice a day, for 2 weeks, followed by a week of no treatment
- every 3 weeks for up to 13 weeks
- every 6 weeks until you finish treatment
- a month after you finish treatment
Hospital visits
- a physical examination
- CT scan or MRI scan
- a hearing test (if you are going to have cisplatin)
- heart trace (ECG)
- blood tests
- urine test
Side effects
- skin rashes, itching and changes to your skin colour
- loose or watery poo (diarrhoea)
- cough
- pain in your joints, back and tummy (abdomen)
- high temperatures
- thyroid problems that can cause tiredness, weight gain, feeling cold and hard infrequent poo
- low levels of salt in your body that may cause you to feel tired, have headaches, muscle cramps and feel sick
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Kai-Keen Shiu
Supported by
Merck, Sharp & Dohme
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040