Please note
This trial is no longer recruiting patients. We hope to add results when they are available.
Head and neck cancers, Laryngeal cancer, Mouth and oropharyngeal cancer, Pharyngeal cancer
Closed
Phase 1/2
This trial is looking at adding certain targeted drugs to radiotherapy for head and neck cancer. Some parts of the trial are also looking at these treatments with immunotherapy.
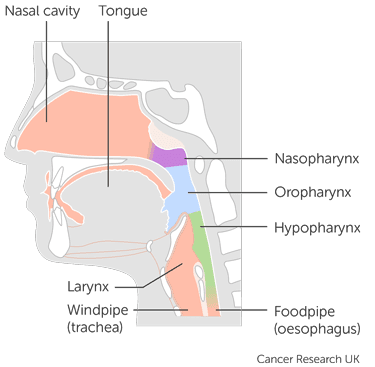
The trial is for people who have one of the following types of head and neck cancer:
cancer of the area that surrounds the voice box ()
cancer of the voice box (laryngeal cancer)
Recruitment start: 5 September 2021
Recruitment end: 23 January 2026
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Dr Anthony Kong
AstraZeneca
University of Birmingham
Last reviewed: 26 Jan 2026
CRUK internal database number: 18055