A trial of giredestrant and Phesgo for breast cancer (heredERA)
Cancer type:
Status:
Phase:
This trial is looking at adding giredestrant to Phesgo to improve treatment for breast cancer.
It is for people whose cancer has grown into surrounding tissues or has spread to other parts of the body. Also, the cancer:
- has tested positive for
HER2 
- has
receptors for the hormone oestrogen 
More about this trial
Breast cancer that has grown into surrounding tissues is  . Breast cancer that has spread to another part of the body is secondary breast cancer. Doctors are looking for ways to improve treatment for these groups of people.
. Breast cancer that has spread to another part of the body is secondary breast cancer. Doctors are looking for ways to improve treatment for these groups of people.
Giredestrant is a new type of hormone treatment. It blocks the hormone oestrogen.
Pertuzumab and trastuzumab are usual treatments for breast cancer that has tested positive for HER2. The combination of trastuzumab and pertuzumab as an injection under the skin is called Phesgo. It works by targeting and blocking the HER2 protein on the breast cancer cell. This stops signals that cancer cells use to divide and grow.
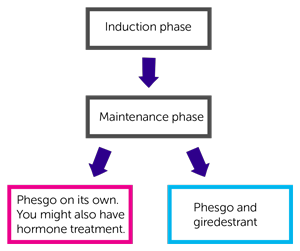
This trial is in 2 parts. The first part is called the induction phase. You will have Phesgo and one of the following chemotherapy drugs:
- docetaxel
- paclitaxel
After the induction phase, you might be able to have long term (maintenance treatment) with one of the following:
- Phesgo with or without hormone treatment
- Phesgo and giredestrant
The main aims of the trial are to find out:
- if having giredestrant and Phesgo works better than Phesgo on its own
- more about the side effects
- how treatment affects quality of life
Who can enter
The following bullet points are a summary of the entry conditions for this trial. Talk to your doctor or the trial team if you are unsure about any of these. They will be able to advise you.
Who can take part
You may be able to join this trial if all of the following apply.
- You have
adenocarcinoma  of the breast that has grown into surrounding tissues and you can’t have surgery to remove it or it has spread elsewhere in the body.
of the breast that has grown into surrounding tissues and you can’t have surgery to remove it or it has spread elsewhere in the body. - You have
HER2  positive breast cancer.
positive breast cancer. - You have cancer that is oestrogen receptor positive (
ER positive breast cancer  ).
). - You have at least one area of cancer that your doctor can measure on a scan.
- If you have had previous treatment for early breast cancer, the cancer came back more than 6 months after your last treatment that didn’t include a hormone treatment.
- You are active but might not be able to do heavy physical work (performance status 0 or 1).
- You have satisfactory blood test results.
- You are willing to use reliable contraception during the trial and for a period after if there is any chance that you or your partner could become pregnant.
- You are at least 18 years old.
As well as the above, to take part in the maintenance part of the trial the following must also apply.
- You have had at least 4 cycles of induction treatment as part of this trial. This is Phesgo alongside either docetaxel or paclitaxel chemotherapy.
- Your cancer didn’t get worse after induction treatment.
- The left side of your heart is working well.
Who can’t take part
Cancer related
You cannot join this trial if any of these apply. You:
- have cancer that has spread to the brain or spinal cord unless it isn’t causing symptoms, has been treated and is stable
- have already had treatment for locally advanced or secondary breast cancer. This doesn’t include hormone treatment. You might be able to join if you had one hormone treatment for breast cancer that has spread or up to 2 cycles of Phesgo (or trastuzumab and pertuzumab) and docetaxel or paclitaxel.
- have already had treatment with fulvestrant or a similar drug
- have already had treatment that targets HER2. You can join if you have had Phesgo, trastuzumab with pertuzumab, trastuzumab alone, ado trastuzumab emtansine, lapatinib, and neratinib before or after surgery.
- have cancer that got worse within 6 months of having trastuzumab, with or without pertuzumab or ado-trastuzumab emtansine after surgery
- have side effects from past treatments unless they are mild. You can join if you have hair loss, pins or needles in your hands or feet (peripheral neuropathy) or other side effects that won’t affect you taking part.
- have had a lot of doses of the chemotherapy drugs called doxorubicin, liposomal doxorubicin, epirubicin or mitoxantrone in the past. Your doctor will know about this.
- have had radiotherapy to help with symptoms within 2 weeks of starting induction treatment
- are taking an experimental drug as part of another clinical trial or took part in another trial within 28 days of starting induction treatment
- have had another cancer that has got worse or needed treatment in the past 5 years unless it has a low chance of coming back. You can join if it was successfully treated
non melanoma skin cancer,  carcinoma in situ (
carcinoma in situ (CIS  ), localised prostate cancer,
), localised prostate cancer, ductal carcinoma in situ of the breast  or early stage womb cancer.
or early stage womb cancer.
Medical conditions
You cannot join this trial if any of these apply. You:
- are short of breath at rest due to your cancer or another health condition and need to have continuous oxygen
- have had treatment with
steroids  for more than 3 months unless it was a low dose
for more than 3 months unless it was a low dose - have high blood pressure that isn’t well controlled
- have a
liver problem 
- have a significant
heart problem  or heart condition that isn’t well controlled with medication. Your doctor checks your heart before you join the trial.
or heart condition that isn’t well controlled with medication. Your doctor checks your heart before you join the trial. - have an increased risk of bleeding, a condition that affects how your blood clots or a blood clot in a vein. This is unless the condition has been treated and is well controlled.
- have had major surgery or a bad injury in the last 14 days
- have HIV or another serious infection that needed treatment in the last 7 days. You can take part if you have HIV that is well controlled with medication. Your doctor will know this.
- are taking medication that affects the CYP enzymes within 2 weeks of starting trial treatment or it hasn't cleared your body
- have had a serious COVID-19 infection within the last 14 days
- have inflammatory bowel disease or any other problem with your bowel that isn’t well controlled
- are sensitive to a hormone treatment called
luteinising hormone blockers  . This applies only to men and women who haven’t been through the
. This applies only to men and women who haven’t been through the menopause  .
. - have any other serious medical condition or mental health problem that the trial team think could affect you taking part
Other
You cannot join this trial if any of these apply. You:
- are allergic to any of the treatments in the trial or anything they contain
- are pregnant or breastfeeding
Trial design
This phase 3 trial is taking place worldwide. The team need to find 821 people to take part including 40 from the UK.
This trial is in 2 parts:
- induction phase
- maintenance phase
Induction phase
Everyone has one of the following treatments:
- Phesgo and docetaxel
- Phesgo and paclitaxel
Your doctor can tell you which treatment you will have.
You have  that last 3 weeks. You have about 4 to 6 cycles. This takes between 3 and 4 ½ months.
that last 3 weeks. You have about 4 to 6 cycles. This takes between 3 and 4 ½ months.
You have Phesgo as an injection into the thigh just under the skin. You have docetaxel or paclitaxel as a drip into a vein.
You have Phesgo once every 3 weeks and:
- docetaxel once every 3 weeks or
- paclitaxel once a week
Treatment takes about 1 to 3 hours each time. You might be at hospital for longer than this.
You can join the maintenance part of the trial if your cancer doesn’t get worse.
Maintenance treatment
This part of the trial is randomised. There are 2 groups. Neither you nor your doctor will be able to decide which group you are in.
You have 1 of the following:
- Phesgo on its own (standard treatment). You might also have hormone treatment
- Phesgo and giredestrant (new combination of treatment)
You have Phesgo as described above.
Giredestrant is a capsule. You take one capsule, once a day, every day. You fill in a diary to record when you take the capsules.
The doctor will talk to you about hormone treatment if they think you need it. This applies only to people having Phesgo on its own.
Everyone has treatment for as long as it is working and the side effects aren’t too bad.
Samples for research
The trial team ask you to give some extra blood samples. Where possible you have these at the same time as your routine blood tests.
They will also ask to look at:
- samples of the cancer from a previous
biopsy  or surgery
or surgery - samples if you have one taken when you finish treatment
The researchers plan to use the samples to:
- look at substances called
biomarkers  to help work out why treatment might work for some people and not for others
to help work out why treatment might work for some people and not for others - find out if your body is making antibodies to giredestrant
- see what happens to giredestrant in the body
They might ask to use any leftover samples for future research. You can say no to this if you don’t want your samples to be used.
Quality of life
The trial team ask you to fill out a questionnaire:
- before you start treatment
- at set times during treatment
The questionnaire asks about side effects and how you’ve been feeling. This is called a quality of life study.
Hospital visits
You see the doctor and have tests before you can take part. These include:
- blood tests
- urine test
- a
physical examination 
- heart trace (ECG)
- heart scan (echocardiogram) or
MUGA scan 
- bone scan
- CT scan or MRI scan
You have treatment at the hospital on the day care ward. You see the doctor every 3 weeks for a check up and blood tests.
Trial scans
You have a bone scan and a CT scan or MRI scan:
- during induction treatment
- 1 month after starting maintenance treatment and then
- every 3 months for the first 3 years and then
- every 4 ½ months until your cancer gets worse
Follow up
You see the doctor a month after you finish treatment. After that, you see them every 3 months for a check up. Or they might call you at home to see how you are. The follow up is every 6 months if you stopped treatment after induction treatment.
Side effects
The trial team monitor you during treatment and afterwards. Contact your advice line or tell your doctor or nurse if any side effects are bad or not getting better.
Giredestrant is a new drug. So there may be side effects we don’t know about yet. The most common side effects we know about so far include:
- pain or stiffness in your joints
- muscle or bone pain
- diarrhoea
- dizziness
- tiredness (fatigue)
- feeling sick
We have information about the following treatments and their side effects:
Your doctor will talk to you about the possible side effects of the treatments before you agree to take part. You’ll have a chance to ask any questions you may have.
Location
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Catherine Harper-Wynne
Supported by
Roche
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040