A trial looking at nimorazole and radiotherapy for head and neck cancer (NIMRAD)
Cancer type:
Status:
Phase:
This trial looked at adding nimorazole to radiotherapy to improve treatment for certain types of head and neck cancer.
It was for people with cancer that may have spread into surrounding tissues but no further.
You pronounce nimorazole as nim-o-ra-zol.
The trial was open for people to join between 2014 and 2019. The team published the results in 2023.
Cancer Research UK supported this trial.
More about this trial
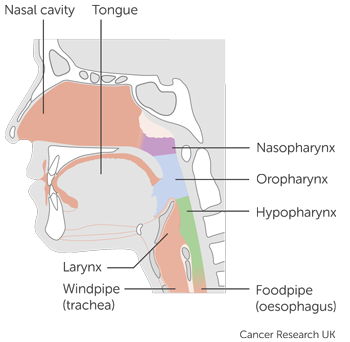
People who took part in this trial had one of the following types of head and neck cancer:
- mouth and oropharyngeal cancer
- cancer of the area that surrounds the voice box (
hypopharynx  )
) - cancer of the voice box (laryngeal cancer)
 was a
was a  for head and neck cancer when this trial was done. Other treatments included chemotherapy or a
for head and neck cancer when this trial was done. Other treatments included chemotherapy or a  .
.
People who took part in this trial couldn’t have chemotherapy or a targeted cancer drug. This may have been because they were older or less fit. So doctors were looking at other treatments to help this group of people. In this trial they looked at having a drug called nimorazole with radiotherapy.
Most cancers have some cells with a low level of oxygen. These are more difficult to kill with radiotherapy than cancer cells with a normal oxygen level. Nimorazole is a drug that gets into the cells with a low level of oxygen. These cells are then more likely to be killed by radiotherapy.
Everyone was put into a treatment group at  :
:
- half had radiotherapy and nimorazole
- half had radiotherapy and an inactive drug (
placebo  )
)
The main aims of this phase 3 trial were to find out:
- if adding nimorazole to radiotherapy improves treatment
- about the side effects of having radiotherapy and nimorazole
- more about
quality of life 
Summary of results
338 people took part in this trial:
- 168 had radiotherapy and nimorazole
- 170 had radiotherapy and an inactive drug
Everyone had radiotherapy for up to 6 weeks. They also had nimorazole or the inactive drug on the days they had radiotherapy.
The team followed everyone for about 3 years. They looked at:
- whose cancer had started to grow again 3 months after they finished radiotherapy
- how long people lived
They didn’t find a difference in either of these between the 2 treatment groups. This applied to everyone who took part, including those who had lower levels of oxygen in their cancer cells.
Side effects
Having nimorazole increased the risk of mild to moderate sickness (nausea). Some people also had more severe sickness. This happened in:
- 17 out of 168 people (10.1%) who had radiotherapy and nimorazole
- 9 out of 170 people (5.3%) who had radiotherapy and the inactive drug
People who had nimorazole had sickness for up to 12 weeks longer compared to those who had the inactive drug.
Quality of life
The team looked at how treatment affected quality of life. They didn’t find a difference in quality of life between the 2 treatment groups.
Conclusion
The team found that adding nimorazole to radiotherapy didn’t improve treatment for this group of people.
Sometimes trials show a different treatment isn’t useful for a particular type or  of cancer. But these trials still add to our knowledge and understanding of cancer and how to treat it.
of cancer. But these trials still add to our knowledge and understanding of cancer and how to treat it.
More detailed information
There is more information about this research in the reference below.
Please note, the information we link to here is not in plain English. It has been written for healthcare professionals and researchers.
Randomised phase III trial of the hypoxia modifier nimorazole added to radiotherapy with benefit assessed in hypoxic head and neck cancers determined using a gene signature (NIMRAD)
D Thomson and others
Journal of Clinical Oncology, 2023. Volume 41, Number 16, supplement
Presented at ASCO 2023 conference, abstract 6006
Randomized Phase 3 Trial of the Hypoxia Modifier Nimorazole Added to Radiation Therapy With Benefit Assessed in Hypoxic Head and Neck Cancers Determined Using a Gene Signature (NIMRAD)
D Thomson and others
International Journal of Radiation Oncology, 2023. Volume 000, Number 00, pages 1-12
Where this information comes from
We have based this summary on the information in the articles above. Some of the information has been reviewed by independent specialists ( ) and published in a medical journal. Some of the information may not have been. We have not analysed the data ourselves. As far as we are aware, the links we list above are active and the articles are free and available to view.
) and published in a medical journal. Some of the information may not have been. We have not analysed the data ourselves. As far as we are aware, the links we list above are active and the articles are free and available to view.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr David Thomson
Supported by
Azanta
Cancer Research UK
NIHR Clinical Research Network: Cancer
The Christie NHS Foundation Trust
The Manchester Clinical Trials Unit
Other information
This Cancer Research UK trial number CRUK/13/006.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



