The research team found that the combination of LCL161 and paclitaxel may be useful for some people with
triple negative breast cancer. But that it did cause some side effects.
This trial was open for people to join between 2013 and 2014. The trial team presented the results at a conference in 2015.
About this trial
This trial recruited 209 women who had been recently diagnosed with triple negative breast cancer.
They were put into one of two treatment groups at
random:
- 103 women had paclitaxel
- 106 women had paclitaxel and LCL161
Everyone taking part had treatment for 12 weeks, and then had an operation to remove their cancer.
Results
The research team looked at how many people had no sign of cancer when they had their operation. Doctors call this a pathological complete response, or pCR.
They found that it was similar in both groups:
- 17 out of 103 women (17%) who had paclitaxel
- 17 out of 106 women (16%) who had paclitaxel and LCL161
They then looked in more detail at women who had a specific combination of
genes. This is called a gene expression signature. About 3 out of 10 women in each group had this genetic signature.
The research team compared women who had this genetic signature with women who didn’t. As before, they looked at how many women had no signs of cancer when they had their operation.
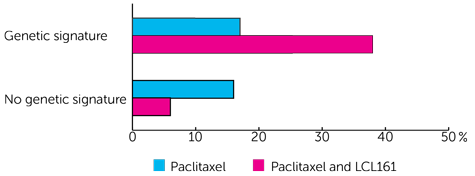
For women who had the genetic signature it was:
- less than 2 out of 10 women (17%) who had paclitaxel
- nearly 4 out of 10 women (38%) who had paclitaxel and LCL161
And for women who didn’t have the genetic signature it was:
- less than 2 out of 10 women (16%) who had paclitaxel
- less than 1 out of 10 women (6%) who had paclitaxel and LCL161
Side effects
Some people taking part in this trial did have side effects. Many of them were mild or didn’t last long. But some people had more serious side effects including a high temperature or lung problems.
Just under 2 out of 10 women (18%) who had paclitaxel and LCL161 had a high temperature (pyrexia), compared to just 1% of those who had paclitaxel.
About 1 out of 10 women (10%) who had paclitaxel and LCL161 had a lung infection (pneumonia), compared to 2% of those who had paclitaxel.
Just under 1 out of 10 women (9%) who had paclitaxel and LCL161 had inflammation of the lung (pneumonitis). No one who had paclitaxel alone had this.
More people who had paclitaxel and LCL161 also had less serious side effects including:
- diarrhoea
- extreme tiredness (fatigue)
- a drop in white blood cells
About 7 out of 10 people in each group had hair loss.
Conclusion
The research team concluded that the combination of paclitaxel and LCL161 may be a useful treatment for people with triple negative breast cancer and a certain combination of genes. But it did cause some serious side effects at the dose used in this trial.
Where this information comes from
We have based this summary on information from the research team. The information they sent us has been reviewed by independent specialists (
peer reviewed 
) but may not have been published in a medical journal. The figures we quote above were provided by the research team. We have not analysed the data ourselves.
 ) but may not have been published in a medical journal. The figures we quote above were provided by the research team. We have not analysed the data ourselves.
) but may not have been published in a medical journal. The figures we quote above were provided by the research team. We have not analysed the data ourselves.