A trial looking at treatment for children and young people with low grade glioma brain tumours (SIOP – LGG 2004 CNS 2004 03)
Cancer type:
Status:
Phase:
This trial looked at adding etoposide to the standard chemotherapy treatment for children and young people with a type of brain tumour called low grade glioma.
It was for children and young people, up to the age of 16, who had a low grade tumour. A low grade tumour means that it usually grows very slowly.
Cancer Research UK supported this trial.
More about this trial

Summary of results
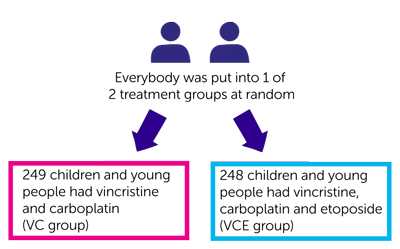
- 249 children and young people had vincristine and carboplatin (VC group)
- 248 children and young people had vincristine, carboplatin and etoposide (VCE group)
- 3 children the cancer disappeared (
complete response  )
) - 59 children the cancer shrunk to at least half the size (
partial response  )
) - 36 children the cancer shrunk to at least a third, but no more than half the size (improvement)
- 98 children the cancer stayed the same (
stable disease  )
) - 15 children the cancer got worse (
disease progression  )
)
- 3 children the cancer disappeared
- 50 children the cancer shrunk to at least half the size
- 33 children the cancer shrunk to at least a third, but no more than half the size
- 106 children the cancer stayed the same
- 18 children the cancer got worse
 in the VCE group. The VC group had more cases of allergic reactions to the treatment.
in the VCE group. The VC group had more cases of allergic reactions to the treatment.  ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Sue Picton
Prof David Walker
Supported by
Cancer Research UK
Cancer Research UK Children's Cancer Trials Team
University of Birmingham
Experimental Cancer Medicine Centre (ECMC)
NIHR Clinical Research Network: Cancer
Other information
This is Cancer Research UK trial number CRUK/05/012.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



