A trial looking at treatment after surgery for HPV positive oropharyngeal cancer (PATHOS)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is looking at less intensive treatment after surgery for oropharyngeal cancer. It is for people whose cancers have tested positive for a virus called HPV (Human Papilloma Virus). This trial is supported by Cancer Research UK.
More about this trial
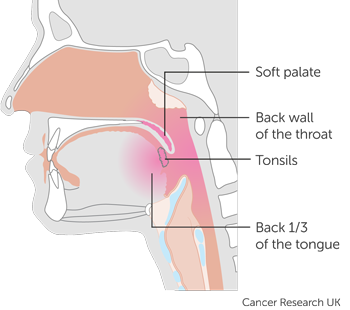
Oropharyngeal cancer is cancer of the middle part of the throat. The areas within the oropharynx include the:
- back third of the tongue
- soft area at the back of the roof of the mouth (the soft palate)
- tonsils and two ridges of tissue in front of and behind the tonsils (called the tonsillar pillars)
- back wall of the throat
Doctors can treat oropharyngeal cancers with surgery followed by treatment to reduce the risk of the cancer coming back (called  ). Adjuvant treatment includes radiotherapy alone or a combination of chemotherapy and radiotherapy (chemoradiation).
). Adjuvant treatment includes radiotherapy alone or a combination of chemotherapy and radiotherapy (chemoradiation).
These treatments can cause short term and long term side effects, such as difficulties with swallowing. Also having chemotherapy and radiotherapy together can make the side effects more severe.
Some oropharyngeal cancers are caused by HPV and doctors know that people with HPV positive cancers of the head and neck respond well to treatment. Researchers want to find out whether this group of patients could have less intensive treatment (called reduced intensity treatment) and as a result have fewer side effects.
This is a phase 3 trial. The aims are to:
- compare the results of people who have less intensive treatment with those who have standard treatment
- find out whether less intensive treatment causes fewer problems with swallowing for people with HPV positive oropharyngeal cancer
Who can enter
You may be able to join this trial if all of the following apply.
- You have squamous cell cancer of the oropharynx
- You have a cancer that tests positive for
HPV 
- You have a cancer that is stage T1,T2 or T3 and you don’t have cancer in your lymph nodes (N0), or if you have cancer in your lymph nodes, these are on the same side of the neck and they measure no more than 6cm across (N1, N2a or N2b). However, if you are a smoker (or have smoked in the last 6 months) and you have N2b cancer, you cannot take part. Vaping does not count as smoking
- Your medical team have decided that your cancer can be removed through the mouth (using a technique called transoral surgery) and a neck dissection
- You are fit and well enough to have surgery followed by radiotherapy
- You are willing to use reliable contraception during treatment if there is any chance that you or your partner could become pregnant
- You are 18 years or older
You cannot join this trial if any of these apply. You
- Have another medical condition that already causes problems with swallowing or is likely to cause problems in the future
- Have oropharyngeal cancer that has spread to other parts of the body and this has been confirmed with scans
- Have had any other cancer in the last 5 years apart from carcinoma in situ of the cervix or non melanoma skin cancer that was successfully treated
- Are pregnant or breast feeding
Trial design
The researchers need 1269 people to join this phase 3 trial.
First of all, everyone has an operation to remove their cancer. You will have the same surgery whether you take part in this trial or not. After your operation the  will look at the cancer under a microscope.
will look at the cancer under a microscope.
The pathologist will look for certain features (such as the stage of the cancer) to help your doctor decide whether you need further treatment and what this treatment may be. Most people diagnosed with your type of cancer will need further treatment after surgery. So don’t be disappointed if this is the case with you.
Group A
If you are in group A, your doctor does not think you will benefit from any further treatment. But you will still have follow up appointments with the trial team.
Group B
If you are in group B, you have radiotherapy. This part of the trial is randomised. The people taking part are put into different treatment groups by a computer. Neither you nor your doctor will be able to decide which group you are in.
- One group have 5 weeks of radiotherapy (at a lower dose)
- The other group have 6 weeks of radiotherapy (standard treatment)
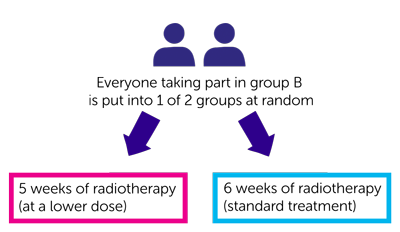
Everyone has radiotherapy once a day from Monday to Friday (with no treatment at the weekend). You usually have this as an out patient and each appointment will take about 20 to 30 minutes. If you have 6 weeks of radiotherapy you have a standard dose of radiotherapy. If you have 5 weeks of radiotherapy, you have a lower dose of radiotherapy and fewer daily treatments (5 weeks instead of 6 weeks).
Group C
If you are in group C you have radiotherapy alone or radiotherapy and chemotherapy. Again this is randomised. Neither you nor your doctor will be able to decide which group you are in.
- One group have radiotherapy alone
- The other group have radiotherapy and chemotherapy
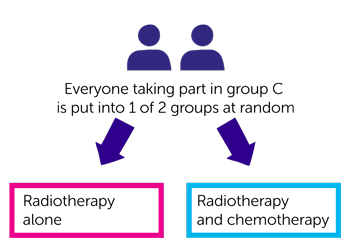
People in both groups have the same dose of radiotherapy. You will probably have chemotherapy with carboplatin or cisplatin through a drip into a vein.
You may have chemotherapy in 1 of 2 ways. You may have it on the first day of your radiotherapy, then again 3 weeks later. Or you have chemotherapy once a week for 6 weeks. The type of chemotherapy you have and how you have it will depend on your hospital policy.
You may stay in hospital overnight or you may have treatment as a day patient. The trial team will give you more detailed information about your treatment plan.
The trial team will ask people in all groups to fill out 3 questionnaires
- Before you start treatment
- 4 weeks after surgery
- 4 weeks after adjuvant treatment (if relevant)
- Then on 3 different occasions over the next 18 months
Each questionnaire will take about 10 to 15 minutes to fill in and it will ask about side effects and how you’ve been feeling.
Hospital visits
You have various tests in preparation for your surgery and adjuvant treatment. For example, you may have a chest X-ray and blood tests. The tests depend on which treatment you have. These would be done as part of your standard care, whether you take part in this trial or not.
The trial team will test a sample of your cancer (taken when you were first diagnosed) to test for the presence of  .
.
As part of this trial you see your doctor regularly for 2 years after finishing your treatment. You have a number of different tests to assess your swallowing. These are done
- Before surgery
- About 4 weeks after surgery
- About 4 weeks after
adjuvant treatment  (if relevant)
(if relevant) - 6, 12 and 24 months after your treatment has finished
After you complete the trial (two years after your treatment is finished) you will continue to see your doctor every year for the next 3 years (until you reach 5 years after treatment). They will check how you are and ask about any side effects.
Side effects
The risks or side effects of an transoral (through the mouth) surgery and a neck dissection include
- Pain
- Infection
- Bleeding
We have more information about surgery for oropharyngeal cancer.
The short term side effects of radiotherapy to the oropharynx include
- Dry mouth
- Taste changes
- Sore and painful mouth and throat
- Sore skin in treatment area
- Pain on swallowing
The late side effects of radiotherapy include
- Difficulty swallowing
- Dry mouth
- Teeth decay
We have more information about the side effects of radiotherapy for oropharyngeal cancers.
The side effects of cisplatin and carboplatin chemotherapy include
- A drop in blood cells causing an increased risk of infection, bleeding problems, tiredness and breathlessness
- Feeling or being sick
- A change to the way your kidneys work. You will have blood tests before and during your treatment to check your kidneys
We have more information about
If you have less intensive treatment, it is possible that the risk of your cancer coming back is greater than those people who have standard treatment. Although the researchers think less intensive treatment will work just as well as standard treatment, this has not been proven by research. This is one of the reasons why the trial is being carried out.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Mererid Evans
Professor Terence Jones
Supported by
Cancer Research UK
Centre for Trials Research
Cardiff University
Velindre University NHS Trust
Other information
This is Cancer Research UK trial number CRUK/13/025
There is more information about this trial on the PATHOS trial website.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



