A trial looking at the treatment of local cancer spread in head and neck cancers
Cancer type:
Status:
Phase:
This trial compared surgery to remove lymph nodes in squamous cell cancers of the head and neck with a ‘watch and wait’ policy using PET-CT scanning.
More about this trial
 is the usual treatment for
is the usual treatment for  cancer of the head and neck that has spread to the lymph nodes. This is usually followed by surgery to remove remaining cancer cells in the
cancer of the head and neck that has spread to the lymph nodes. This is usually followed by surgery to remove remaining cancer cells in the  in the neck. This is called a neck dissection.
in the neck. This is called a neck dissection.
Some people don’t have any cancer in their lymph nodes after chemoradiation and don’t need surgery. But when this trial was done, there was no reliable way to identify which people still had remaining cancer cells. Doctors thought a new type of scanner called a PET-CT could help to see if there were any cancer cells in the lymph nodes.
The aims of the trial were to
- find out if PET-CT scanning after chemoradiation could help this group of people
- find out more about
quality of life  and cost
and cost
Summary of results
The trial team found that PET-CT scanning was very useful. It can prevent unnecessary operations and better target areas of cancer in the small number of people who do need surgery.
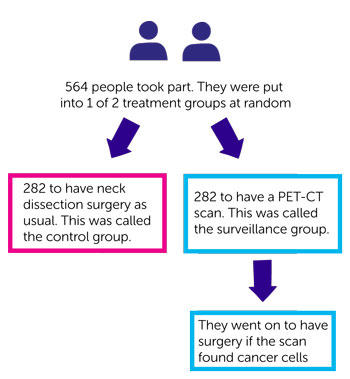
564 people took part. After chemoradiation, they were put into 1 of 2 treatment groups at random
- 282 to have neck dissection surgery as usual. This was called the control group
- 282 to have a PET-CT scan. This was called the surveillance group. They went on to have surgery if the scan found cancer cells.
The trial team looked at who had surgery. This was
- 221 people in the control group
- 54 people in the surveillance group
They compared
- the number of people living 2 years after treatment had finished. This is called overall survival
- quality of life
They found there was no difference between the 2 different groups in either of these.
The researchers concluded that
- there was no difference in overall survival between the control group and the surveillance group
- having a PET-CT resulted in fewer operations and this was more cost effective
We have based this summary on information from the research team. The information they sent us has been reviewed by independent specialists ( ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Hisham Mehanna
Supported by
Experimental Cancer Medicine Centre (ECMC)
NIHR Clinical Research Network: Cancer
NIHR Health Technology Assessment (HTA) programme
University Hospitals Coventry and Warwickshire
University of Warwick
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



