A trial looking at photochemical internalisation (PCI) for advanced bile duct cancer
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is looking at a new treatment called photochemical internalisation followed by chemotherapy for bile duct cancer (cholangiocarcinoma). It is for people whose bile duct cancer cannot be removed with surgery. The cancer may have spread into surrounding tissue, or elsewhere in the body.
More about this trial
Doctors usually treat advanced cancer of the bile duct with chemotherapy drugs such as gemcitabine and cisplatin. Chemotherapy may help to shrink or slow the growth of the cancer for a time, but it can start to grow again. Researchers are looking for new treatments and to improve existing treatments.
In this trial, researchers are looking at photochemical internalisation (PCI). With this treatment, you have 2 drugs. These are Amphinex (which makes body tissue sensitive to light) and a type of chemotherapy called gemcitabine.
After your treatment with Amphinex and gemcitabine, the doctor directs a laser light through a fibre directly onto the cancer via an endoscope. The light activates the Amphinex, which in turn helps the gemcitabine to get into the cancer cells and kill them.
The aims of this study are to
- Find the best and safest dose of the combination of Amphinex and laser light
- Learn more about the side effects
- Find out if photochemical internalisation and chemotherapy is better than chemotherapy alone for bile duct cancer
Who can enter
You may be able to join this trial if all of the following apply.
- You have adenocarcinoma of the bile duct (cholangiocarcinoma) that cannot be removed with surgery. Your cancer may have spread into surrounding tissue, or elsewhere in the body.
- Your cancer started in the bile duct outside of the liver (extrahepatic bile duct cancer)
- Your bile ducts are draining at least 50% of bile out of the liver into the bowel (your doctors can advise you about this)
- You have satisfactory blood and urine test results
- You are willing to use reliable contraception during the trial and for 6 months afterwards if there is any chance that you or your partner could become pregnant
- You are well enough to carry out all your normal activities, apart from heavy physical work (performance status of 0, 1 or 2)
- You are at least 18 years old
You cannot join this trial if any of these apply. You
- Have already had treatment for your bile duct cancer
- Are already taking part in another trial looking at a treatment
- Have had any other cancer, unless it was a very early stage and has been successfully treated
- Cannot have a CT scan or MRI scan for any reason
- Have a known allergy or sensitivity to drugs that make the body tissues sensitive to light (photosensitisers)
- Need to have any surgery, an endoscopy or dental treatment in the 4 weeks following your treatment in this trial
- Have certain heart problems (the trial team can advise you about this)
- Have primary sclerosing cholangitis
- Have ataxia telangiectasia or porphyria
- Are likely to need an eye examination using a slit-lamp in the 3 months following treatment in this trial. The slit-lamp is a machine that uses intense light to look into the eye
- Have significant hearing problems
- Are taking an anti epileptic drug called phenytoin
- Have any other serious medical condition or mental health problem that the trial team think could affect you taking part
- Are pregnant or breastfeeding
Trial design
This phase 1/2 trial needs about 61 people to join. This trial has 2 parts.
In part 1, the first few patients joining will have the lowest dose of Amphinex and low laser light energy. If they don’t have any serious side effects, the next few patients will have a higher dose of both Amphinex and the laser light. And so on, until the doctors find the best dose to give. This is called a dose escalation study.
The dose of gemcitabine may also vary. If it is difficult to find a safe dose of Amphinex with the first patients, some people may have a lower dose of gemcitabine. Your dose of Amphinex, red light and gemcitabine depends on when you join the trial.
Everyone in part 1 needs to have a  put into their bile duct in order to have treatment. This is done during a procedure called an endoscopy. You may have sedation before you have an endoscopy to make you feel drowsy. You may stay in hospital that night.
put into their bile duct in order to have treatment. This is done during a procedure called an endoscopy. You may have sedation before you have an endoscopy to make you feel drowsy. You may stay in hospital that night.
If you already have a stent, the trial team will need to check this is in the right position during endoscopy.
On the first day of your Photochemical Internalisation (PCI) treatment, you have Amphinex as an injection into your vein. 4 days later you have gemcitabine. You have this through a drip into your vein. This takes about 30 minutes. About 3 hours later you have the laser light therapy via an endoscopy. You may have sedation, or you may have a  . You will also be given painkillers.
. You will also be given painkillers.
You stay in hospital for about 7 days. You have regular tests during this time, including blood tests and monitoring of your heart rate, blood pressure and temperature. You will be asked to provide a stool sample every day if possible. The trial team will check whether you have any pain and make sure you have regular pain killers if you need them.
As Amphinex is activated by light your skin and eyes will become sensitive to light for a time after you have it. Normal light can also activate Amphinex so you will need to stay in a room with only low lighting for the first 2 weeks. You can slowly increase the amount of light as the days go by.
The trial team will give you instructions on how to protect yourself and how much light you can be exposed to until things return to normal, which may take up to 3 months.
You will be provided with a light meter to be able to read the light intensity. The trial team will ask you to fill in a photosensitivity questionnaire every day for about 1 month.
Between 1 and 3 weeks after PCI treatment you have combination chemotherapy using the drugs gemcitabine and cisplatin. You have both drugs through a drip into your vein. This takes about 1 ½ hours. You have treatment once a week for the first 2 weeks, then no treatment in the third week. Each 3 week period is called a cycle of treatment and you can have up to 8 cycles.
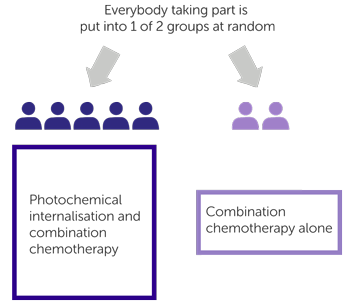
The second part of the trial is randomised. The people taking part are put into treatment groups by a computer. Neither you nor your doctor will be able to decide which group you are in, but you will know which treatment you are due to have.
- One group have photochemical internalisation (PCI) and combination chemotherapy
- The other group have combination chemotherapy alone (this is the
control group  )
)
For every 7 patients entered into the trial, 5 people have PCI and chemotherapy and 2 people have chemotherapy alone.
If you have PCI, you have the best dose of Amphinex, gemcitaibine and laser light treatment found in the first part of the trial. You have PCI in the same way as people in part 1. You stay in hospital for about the same amount of time and have the same tests as in part 1. You start combination chemotherapy about 3 weeks after your PCI treatment.
If you have chemotherapy alone, you start this within 3 weeks of joining the trial. You may have a stent put into your bile duct if your doctor thinks you need one.
Both groups have combination chemotherapy as described in part 1.
Hospital visits
You will see the doctors and have some tests before you start treatment. The tests include
- Physical examination and assessment of your general health and any medicines you are taking
- Blood tests
- Urine test
- Heart trace (
ECG  )
) - Tests to check that your kidneys are working
- A CT scan or MRI scan if you have not had one in the last 2 weeks
You will need to have a sample ( ) taken of your cancer to confirm your diagnosis if this has not been done recently.
) taken of your cancer to confirm your diagnosis if this has not been done recently.
If you have PCI you stay in hospital for about 7 days where you are monitored quite closely, including regular blood tests.
During combination chemotherapy you go to hospital once every week for the first 2 weeks of each 3 week cycle. You don’t usually need to go to hospital on the 3rd week of each cycle. When you go to hospital for your treatment, the doctors will check how you are. You will also have regular blood tests.
When you finish treatment, you may have a scan of your cancer. You will have a check up and further scan every 3 months for between 6 or 15 months depending on which part of the trial you take part in. After that, the trial team may continue to contact you (or your GP) every 3 months to ask how you are doing.
Side effects
Doctors routinely use drugs similar to Amphinex, but Amphinex has only been used in a small number of patients so far. This is the first time Amphinex has been used in combination with gemcitabine, so there may be side effects we don’t know about yet.
A common side effect of Amphinex is that you become sensitive to light. You may have one or more of the following skin reactions
- Redness
- Sunburn
- Blistering
- Burns
- Darkening
The trial team will give you specific instructions about how to protect yourself to try to prevent these problems.
Side effects of PCI treatment may also include
- Pain where you had the treatment
- Redness and swelling of the tissue treated and healthy tissue nearby which could lead to bleeding, infection, crusting and damage to the skin
- Fever
- Constipation
- Feeling or being sick
- A drop in blood cells causing an increased risk of infection, bleeding problems, tiredness and breathlessness
- Feeling dizzy
The side effects of gemcitabine include
- A drop in blood cells causing an increased risk of infection, bleeding problems, tiredness and breathlessness
- Feeling short of breath or wheezy soon after you have the drug. Your nurse will keep a close eye on you and treat symptoms if you need it
- Feeling or being sick
- Changes to the way your liver works. You have regular blood tests to check this, any changes usually go back to normal when treatment finishes
- Skin rash
- Small amount of blood and protein in your urine may be found when the nurse tests your urine. This is not harmful
- Flu like symptoms
We have information about the combination chemotherapy of gemcitabine and cisplatin.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Richard Sturgees
Supported by
PCI Biotech
Quanticate
Theradex
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040