A trial looking at how food affects ceritinib in people with non small cell lung cancer
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is for people with non small cell lung cancer that has spread and has a change in the ALK protein.
More about this trial
Changes to the ALK (anaplastic lymphoma kinase) protein can send signals to cancer cells telling them to grow. Ceritinib is a targeted cancer drug (a biological therapy) called a growth blocker. It works by blocking signals that tell the cancer cells to grow and divide
You take ceritinib as a capsule on an empty stomach. But research suggests it might be better to have it with a low fat meal. So taking a lower dose might have the same benefit and the side effects might be less.
In this study some people will take ceritinib on an empty stomach and some will take it with a low fat meal. The team will also look at 2 different doses of ceritinib with a low fat meal.
The main aim of the study is to find what effect dose and food has on ceritinib for people with non small cell lung cancer with a change in the ALK protein.
Who can enter
The following bullet points list the entry conditions for this study. Talk to your doctor or the study if you are unsure about any of these. They will be able to advise you.
You may be able to join this study if all of the following apply
- You have non small cell lung cancer that is stage 3b or stage 4 and has a change (mutation) in the ALK protein
- If your cancer has come back (relapsed) within a year of finishing your initial treatment and you haven’t had any treatment for the relapsed cancer
- If you haven’t had treatment for your advanced NSCLC before
- You must have at least 1 area of cancer that can be measured on a
scan 
- You are well enough to be up and about for at least half the day (performance status 0, 1 or 2)
- You have satisfactory blood test results
- You are willing to reduce your alcohol intake to 1 drink a day for the 3 days before having blood samples taken as part of the study
- You are willing not to eat or drink the juice of grapefruit, pomegranate, starfruit and Seville orange for the 3 days before having blood samples taken as part of the study
- You are willing to use reliable contraception during treatment and for 3 months afterwards if there is any chance you or your partner could become pregnant
- You are at least 18 years old
You cannot join this study if any of these apply. You
- Have cancer spread to the brain or spinal cord unless there are no symptoms and you don’t need to have your dose of steroids increased within 2 weeks of starting treatment
- Have had any other treatment within 2 weeks of starting treatment in this study. For
nitrosoureas  and mitomycin C you must have had them at least 6 weeks before starting treatment
and mitomycin C you must have had them at least 6 weeks before starting treatment - Still have side effects from any previous treatment apart from hair loss, skin and nail changes and mild peripheral neuropathy
- Have had another cancer in the past 3 years apart from
non melanoma skin cancer  that has been completely removed by surgery and
that has been completely removed by surgery and carcinoma in situ  of any kind that has been completely removed by surgery
of any kind that has been completely removed by surgery - Have had radiotherapy to the lungs within 4 weeks of starting treatment. For radiotherapy to another part of the body and to relieve symptoms of bone pain it is 2 weeks
- Still have side effects from having radiotherapy
- Have had heart problems such as angina or heart attack, in the past 6 months
- Have congestive heart failure
- Have high blood pressure that isn’t controlled by medication
- Have had a disease affecting the lung, such as interstitial lung disease or pneumonia caused by radiotherapy, that affects your daily life
- Have a problem, such as ulcerative colitis, Crohn’s disease, being sick or diarrhoea, that could affect how your gut absorbs ceritinib
- Have had inflammation of the pancreas (pancreatitis) or an increase in the pancreatic enzymes amylase or lipase due to a disease of the pancreas
- Are having certain medications that affect how the heart works or body substances called CYP enzymes unless they can be stopped at least 1 week before starting treatment
- Are taking warfarin or similar medication that affects how your blood clots
- Are taking steroids and the dose keeps changing or increasing. If you are taking steroids to replace hormones in the body or for symptom control you might be able to take part. The dose of these steroids must be stable or decreasing within 5 days of starting treatment
- Are taking medication to control fits (seizures), such as carbamazepine or phenytoin, unless it can be stopped a week before starting and during the study
- Are sensitive to ceritinib or its ingredients
- Have had major surgery within 4 weeks of starting treatment apart from video assisted thoracic surgery (VATS) and mediastinoscopy
- Have any other medical or mental health condition that the study team think could affect you taking part
- Are pregnant or breastfeeding
Trial design
This is an international phase 1 study. The team need 300 people from around the world to join. At least 6 people will come from the UK. Everyone will have ceritinib.
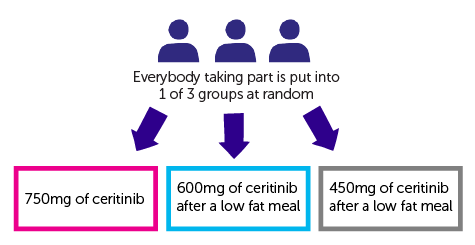
It is a randomised study. People are put into 1 of 3 groups. Neither you nor your doctor can choose which group you are in.
- 750 mg of ceritinib (
standard treatment  )
) - 600 mg of ceritinib after a low fat meal
- 450 mg of ceritinib after a low fat meal
Ceritinib is a capsule you take once a day.
You take the 750 mg dose in the morning on an empty stomach. You must not eat or drink apart from water for an hour before or 2 hours after taking ceritinib.
You take the 450 mg and 600 mg doses immediately after (within 30 minutes) of your low fat breakfast. The study team will give you examples of what a low fat meal is. You have a diary to record your meals. You take this to each hospital appointment.
You continue taking ceritinib as long as it is helping and the side effects aren’t too bad.
Tissue and blood samples
The researchers will ask for a sample of your cancer that was removed when you had surgery or a  and for some extra blood samples.
and for some extra blood samples.
The team will use these samples to look for substances (biomarkers) to help understand who might benefit most from ceritinib. They will also at the pharmacogenetics. This how the genes affect the way people respond to ceritinib.
The team will ask for another sample of tissue if your cancer gets worse. You don’t have to agree to this.
Hospital visits
You see the doctor before taking part to have some tests. These include
During treatment you see the doctor regularly for a physical examination and blood tests. You have a CT scan or MRI scan 9 weeks after starting treatment and then every 6 weeks.
Side effects
The most common side effects of ceritinib are
- diarrhoea or constipation
- feeling or being sick
- tiredness
- tummy (abdominal) pain
- loss of appetite
- not having enough water in the body (
dehydration  )
) - rash
- a change to the way the kidneys and liver works
Your doctor will talk to you about the side effects before you agree to take part.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Alastair Greystoke
Supported by
Experimental Cancer Medicine Centre (ECMC)
Novartis
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



