A trial looking at different doses of aspirin to prevent cancer in people who have Lynch syndrome (CaPP3)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is comparing 3 different doses of aspirin as a way of stopping cancer developing in people who have Lynch syndrome.
Cancer Research UK supports this trial.
More about this trial
People who have Lynch syndrome (also known as HNPCC) have inherited a faulty version of a gene, which increases their chances of developing certain cancers including bowel cancer and womb cancer.
A trial called CAPP2 showed that aspirin reduces the risk of cancer in people with Lynch syndrome. But in a small number of people, aspirin can cause bleeding in the stomach. This happens more often when people take higher doses of aspirin. So researchers want to see if lower doses of aspirin work as well.
The aim of this trial is to find the best dose of aspirin that people with Lynch syndrome should take to prevent cancer.
Who can enter
You may be able to join this trial if all of the following apply. You
- Have Lynch syndrome (HNPCC)
- Are able to swallow tablets
- Are at least 18 years old
You cannot join this trial if any of these apply. You
- Regularly take a non steroidal anti inflammatory drug. For example you have had 3 doses a week, for more than 3 months in the last 3 years
- Have taken 150 mg or more of aspirin every day for more than 3 months in the last 3 years
- Are known to be very sensitive to aspirin
- Have problems with your liver or kidneys
- Have had an ulcer in your food pipe, stomach or bowel (a peptic ulcer) in the last 3 months
- Have an increased risk of bleeding or take drugs to thin your blood (anticoagulants)
- Have changes (mutations) in certain genes that are known as constitutional mismatch repair deficiency
- Have any other medical condition that could affect you taking part (if you have high blood pressure, you will need to have this treated before you start taking aspirin)
- Are pregnant or planning on becoming pregnant in the next 2 years
- Are breastfeeding
Trial design
This is an international phase 3 trial. The researchers need about 2000 people to take part worldwide and hope that around 1500 people from the UK will take part. Everybody joining the trial will take aspirin tablets each day.
This trial is in 2 parts.
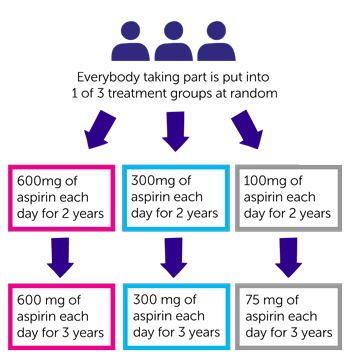
Part 1 of trial is randomised. The people taking part are put into 1 of the following treatment groups by a computer:
- 600mg of aspirin each day
- 300mg of aspirin each day
- 100mg of aspirin each day
Neither you nor your doctor will be able to decide which group you are in. Also, whatever dose of aspirin you take, the tablets will look the same, so neither you nor your doctor will know which group you are in. This is called a double blind trial.
You take aspirin for 2 years. Then you see the trial doctor and find out which dose of aspirin you have had. This is part 2 of the trial.
In part 2 you continue taking aspirin for 3 more years. You take:
- 600 mg of aspirin each day if you have had 600 mg during part 1
- 300 mg of aspirin each day if you have had 300 mg during part 1
- 75 mg of aspirin each day if you have had 100 mg during part 1
The researchers expect the trial to finish in 2024.
The trial team will contact you by phone after 3 months, 6 months, a year and 18 months. They will check how you are getting on and ask some questions about your health. The phone calls should take no longer than 20 minutes. After 2 years of treatment, they will phone you once a year.
You can also contact your research nurse or doctor if you have any problems or questions.
The trial team will look at your medical records. All the information they collect about you will be kept confidential.
Hospital visits
You will see the trial team before you join the trial. If you agree to take part, you have a blood test. You start taking aspirin shortly afterwards. The tablets will be delivered to your home every 6 months.
You see the trial team again after 2 years and 5 years. The researchers will ask you to give more blood samples at these visits.
Side effects
The most serious side effect of aspirin is bleeding. Other possible side effects include
- Tummy (abdominal) pain
- Feeling or being sick
- Heartburn
- Diarrhoea
- Blood in the stool or black stools
Very rarely people have an allergic reaction to aspirin. And it can sometimes make asthma worse.
It is important to remember that millions of people around the world take aspirin with very few side effects. The trial team will talk to you about all the possible risks before you agree to join.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Sir John Burn
Supported by
Bayer Pharma AG
Cancer Research UK
NIHR Clinical Research Network: Genetics
Newcastle University
Newcastle upon Tyne Hospitals NHS Foundation Trust
Other information
This is Cancer Research UK trial number CRUK/12/039.
There is more information on the CaPP3 website.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



