A trial looking at chemotherapy and ADI-PEG 20 for mesothelioma (ATOMIC-Meso)
Cancer type:
Status:
Phase:
This trial looked at adding a drug called ADI-PEG 20 to standard chemotherapy for mesothelioma.
It was for people with mesothelioma that started in the covering of the lungs (pleura).
The trial was open for people to join between 2017 and 2021. The team published the results in 2024.
More about this trial
Mesothelioma can start in the covering of the lungs or the tummy (abdomen). Pleural mesothelioma is when it starts in the tissues covering the lungs.
Whenever possible doctors treat pleural mesothelioma with surgery but some people can’t have surgery. This is because the cancer has grown too far. This means it is  . People with advanced mesothelioma might have chemotherapy instead.
. People with advanced mesothelioma might have chemotherapy instead.
Pemetrexed and cisplatin chemotherapy was a  for pleural mesothelioma when this trial was done. Doctors were looking at ways to improve treatment. In this trial they looked at ADI-PEG 20.
for pleural mesothelioma when this trial was done. Doctors were looking at ways to improve treatment. In this trial they looked at ADI-PEG 20.
Researchers had found a new way of getting rid of cancer cells. This was by removing a substance called arginine. Arginine helps with different jobs in the body, including cell growth. Doctors thought that if you removed arginine, cancer cells would not be able to replace it. And this might stop the cancer cells from growing. We know from earlier research that ADI-PEG 20 could remove arginine from cells. ADI-PEG 20 is an injection you have into a muscle.
This was a phase 2 and 3 trial. Those taking part had one of the following:
- pemetrexed and cisplatin chemotherapy with a dummy drug (
placebo  )
) - pemetrexed and cisplatin chemotherapy with ADI-PEG 20
The main aims of this trial were to find out:
- if adding ADI-PEG 20 to chemotherapy improves treatment for advanced mesothelioma
- more about the side effects
Summary of results
A total of 249 people were put into a treatment group at random:
- 124 had standard chemotherapy and a dummy drug
- 125 had standard chemotherapy and ADI-PEG 20
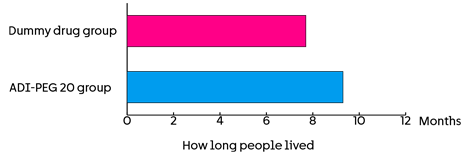
The team followed everyone up for about a year. They looked at how long people lived. This was:
- 7.7 months in the chemotherapy and dummy drug group
- 9.3 months in the chemotherapy and ADI-PEG 20 group
They also looked at how long before the cancer started to grow again. This was:
- 5.6 months in the chemotherapy and dummy drug group
- 6.2 months in the chemotherapy and ADI-PEG 20 group
The committee that monitors the safety and design of the trial did an early analysis of the results. This was to check how treatment was working. The results showed that chemotherapy and ADI-PEG 20 were working better than chemotherapy and the dummy drug. So the trial stopped recruiting people earlier than planned.
Side effects
Some of the side effects of treatment were mild and didn't last long.
The most common side effects of chemotherapy and the dummy drug included:
- feeling sick
- tiredness (fatigue)
- weight loss
The most common side effects of chemotherapy and ADI-PEG 20 included:
- feeling sick
- tiredness
- constipation
Some people had more severe side effects. This was:
- 21 out of 124 (17%) in the chemotherapy and the dummy drug group
- 36 out of 125 (29%) in the chemotherapy and ADI-PEG 20 group
The more severe side effect for both groups was a drop in the number of blood cells. This caused an increased risk of infection, bruising or bleeding, tiredness or breathlessness. This was worse in the group who had chemotherapy and ADI-PEG 20.
A few people in the chemotherapy and ADI-PEG 20 group (2%) also had severe side effects that included:
- skin rash
- an allergic reaction to treatment
Nobody in the chemotherapy dummy drug group had these side effects.
Conclusion
The team found that chemotherapy and ADI-PEG 20 helped some people live a bit longer. This was compared to those who had chemotherapy and the dummy drug.
The team say this research proves that ADI-PEG 20 alongside pemetrexed and cisplatin chemotherapy is a useful treatment option for advanced mesothelioma.
The team suggest that more research is done looking at ADI-PEG 20 for other types of cancer that are difficult to treat.
More detailed information
There is more information about this research in the reference below.
Please note, the information we link to here is not in plain English. It has been written for healthcare professionals and researchers.
Pegargiminase Plus First-Line Chemotherapy in Patients With Nonepithelioid Pleural Mesothelioma The ATOMIC-Meso Randomized Clinical Trial
Peter W. Szlosarek and others
JAMA Oncology, 2024. Volume 10, Issue 4, pages 475-483
Where this information comes from
We have based this summary on the information in the article above. This has been reviewed by independent specialists ( ) and published in a medical journal. We have not analysed the data ourselves. As far as we are aware, the links we list above are active and the article is free and available to view.
) and published in a medical journal. We have not analysed the data ourselves. As far as we are aware, the links we list above are active and the article is free and available to view.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Peter Szlosarek
Supported by
Polaris Pharmaceuticals Inc
Queen Mary University of London
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040