A trial of resminostat for T cell lymphoma of the skin (RESMAIN)
Cancer type:
Status:
Phase:
This trial looked at resminostat for T cell lymphoma of the skin. It was for people whose lymphoma hadn’t got worse after their last treatment.
The trial was open for people to join between 2017 and 2022. The team presented the results at a conference in 2023.
More about this trial
Cutaneous T cell lymphoma (CTCL) is a rare type of lymphoma that affects the skin. The most common types are:
- mycosis fungoides
- Sezary syndrome
When this trial was done, doctors could use different treatments for T cell lymphoma of the skin. But it can get worse despite this. So doctors were looking for ways to improve treatment.
In this trial, researchers looked at a drug called resminostat. It was a new drug when this trial was done. Doctors thought it might help people with advanced skin lymphoma. But they weren’t sure, so they wanted to find out more.
Resminostat is a drug that blocks substances ( ) in the body called histone deacetylases. You pronounce this as dee-as-et-isle-azes. Cancer cells need these enzymes to grow and divide. So blocking them may help to stop cancer growing.
) in the body called histone deacetylases. You pronounce this as dee-as-et-isle-azes. Cancer cells need these enzymes to grow and divide. So blocking them may help to stop cancer growing.
In this trial:
- half had resminostat
- half had a dummy drug (
placebo  )
)
Everyone had treatment for as long as it was working and the side effects weren’t too bad. When the lymphoma got worse on the dummy drug, they stopped taking it and switched to having resminostat.
The main aims of this trial were to:
- see if resminostat prevents or delays skin T cell lymphoma coming back or getting worse
- learn more about the side effects
- find out about
quality of life 
Summary of results
201 people took part in this trial. Of those:
- 164 had mycosis fungoides
- 37 had Sezary syndrome
They all had skin lymphoma that hadn’t got worse after:
- their last treatment to the whole body (
systemic treatment  ) or
) or - after having a type of radiotherapy called total skin electron beam (TSEB)
Everyone was put into a treatment group at random:
- 100 people had resminostat
- 101 people had a dummy drug
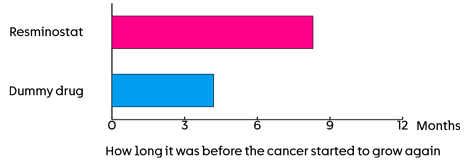
The team looked at how long it was before the cancer started to grow again. This was about:
- 8.3 months in those who had resminostat
- 4.2 months in those who had the dummy drug
The team say resminostat seemed to work a bit better for people who had mycosis fungoides.
The team also looked at:
- the length of time before itching got worse
- how long people lived
- quality of life
They found no difference in any of these between the 2 treatment groups.
Side effects
There weren’t any unexpected side effects of resminostat. The most common side effects of resminostat were:
- feeling or being sick
- diarrhoea
The team found that these side effects were manageable.
Conclusion
The trial team found that resminostat increased the length of time before the cancer started to grow again. The team plan to look at the longer term results. We hope to add those results to this summary when they are available.
Where this information comes from
We have based this summary on information from the research team. The information they sent us has been reviewed by independent specialists ( ) but may not have been published in a medical journal. The figures we quote above were provided by the research team. We have not analysed the data ourselves.
) but may not have been published in a medical journal. The figures we quote above were provided by the research team. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Julia Scarisbrick
Supported by
4SC AG
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040