A study looking at giving a higher dose of radiotherapy to patients with lung cancer (ARTFORCE PET-boost)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This study is looking at whether a higher dose of radiotherapy guided by a PET scan is better at stopping a non small cell lung cancer from coming back or growing.
A new targeted type of radiotherapy called IMRT will be used to treat the lung cancer.
More about this trial
Radiotherapy, either with chemotherapy or on its own is the usual treatment for non small cell lung cancer. But there is still a risk of the cancer coming back or getting worse after treatment.
Doctors want to see if it is better to treat all the cancer with the higher dose or whether they can just increase the dose to an area of ‘active’ cancer. This means an area of cancer where there are lots of cell changes. They will find this area of cancer using a PET-CT scan. They also want to see if this will improve the way the radiotherapy is given.
IMRT allows the radiotherapy beams to be shaped more accurately and so may avoid more of the surrounding normal tissue. This means a higher dose can be given.
People with non small cell lung cancer usually have a set dose of radiotherapy. In this study the researchers will also work out the dose of radiotherapy for everyone individually, rather than just give the standard dose.
The aims of the study are to find out
- if it is practical to give a targeted individual dose of radiotherapy
- what the side effects are
- if giving this treatment to all or part of the cancer is better at stopping it from continuing to grow or coming back
- if the treatment can help people with lung cancer to live longer
Who can enter
The following bullet points list the entry conditions for this study. Talk to your doctor or the study team if you are unsure about any of these. They will be able to advise you.
You may be able to join this study if all of the following apply. You
- Have been diagnosed with non small cell lung cancer that has not spread elsewhere in the body and your cancer can’t be removed with surgery
- Have satisfactory blood results
- Are well enough to be up and about for at least half the day (performance status 0, 1 or 2)
- Have satisfactory breathing test results (lung function tests)
- Are willing to use reliable contraception during treatment and up to 6 months afterwards if there is any chance that you could become pregnant. If you are a man taking part you will need to use reliable contraception if you are having chemotherapy as part of your treatment.
- Are at least 18 years old
You cannot join this study if any of these apply. You
- Have cancer spread to a main blood vessel unless it is contained
- Have had radiotherapy to your chest
- Have had any other cancer in the last 3 years, apart from very early cancers (in situ carcinomas) or non melanoma skin cancer
- Have a condition called superior vena cave syndrome
- Have fluid around your heart or in your lungs caused by your cancer
- Are pregnant or breast feeding
Trial design
This is an international phase 2 study. The researchers need 164 people to take part and hope 15 to 24 people will be in the UK.
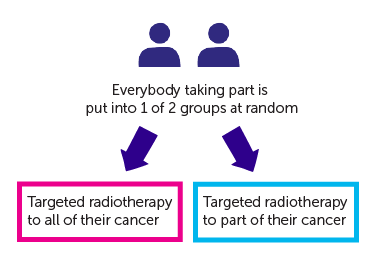
It is a randomised study. The people taking part are put into 1 of 2 treatment groups by a computer. Neither you nor your doctor will be able to decide which group you are in.
- one group have targeted radiotherapy to all of their cancer
- the other group have targeted radiotherapy to part of their cancer
Everyone will have treatment Monday to Friday, once a day, for 32 days.
You might have chemotherapy alongside the radiotherapy. If you do, the doctors will explain what drugs you will have and when you have them.
Both groups have a higher radiotherapy dose given to their tumours compared to the standard treatment dose.
Quality of life
The study team will ask you to fill out 2 different questionnaires before you start treatment, and then every time you see the doctors after you finish treatment. The questionnaires will ask about side effects and how you’ve been feeling. This is called a quality of life study.
Hospital visits
You will see the doctors and have some tests before you start treatment. The tests include
- Blood tests
- A physical examination
- Heart trace (
ECG  )
) - Lung function tests
- Bronchoscopy
- CT scan of your chest and
abdomen 
- PET- CT scan
Some people might also have a
Before you start your treatment the doctors will plan your radiotherapy.
You need to go to hospital once a day Monday to Friday for your treatment. It will be given over 32 days. The treatment takes about 15 minutes.
After your treatment has finished you see the doctors once a week. When any side effects are better you then see them after 4 weeks and then at 3, 6, 12 and 18 months. They will examine you and check how you are.
You also have
- a CT scan every 3 months for 18 months
- a PET-CT scan at 3 months and then 1 year
Side effects
You may have side effects from radiotherapy to the chest. The most common are
- cough
- tiredness
- pain on swallowing
- shortness of breath - this can start towards the end of your radiotherapy or after radiotherapy has finished and may last for up to 6 weeks
- reddening and soreness of the skin in the treatment area
We have more information about side effects of radiotherapy to the chest
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor C Faivre-Finn
Supported by
Experiment Cancer Medical Centre (ECMC)
Netherlands Cancer Institute
EU Seventh Framework Programme (FP7)
The Christie NHS Foundation Trust
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



