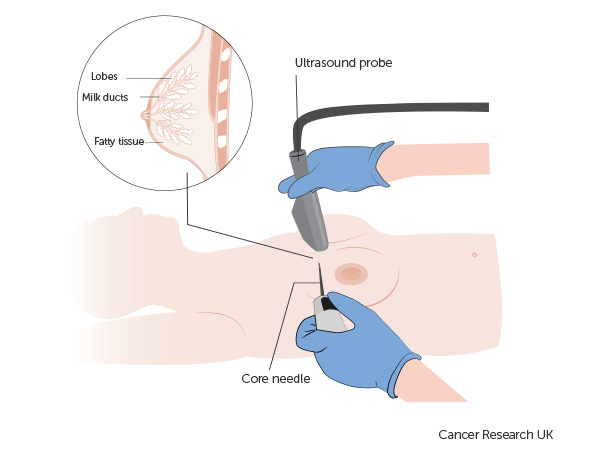
A study looking at ultrasound guided biopsies for breast cancer (NOSTRA)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
- have large amounts of a protein called human epidermal growth factor receptor (HER2 positive)
- don’t have receptors for the hormone oestrogen (oestrogen receptor negative or ER negative)
More about this trial
- a combination of chemotherapy drugs called FEC with pertuzumab (Perjeta) and trastuzumab (Herceptin). You then have docetaxel with pertuzumab and trastuzumab
- FEC and then docetaxel with pertuzumab and trastuzumab
- docetaxel and carboplatin, and then pertuzumab and trastuzumab
- still has cancer cells after neoadjuvant treatment (residual disease)
- doesn’t have cancer cells after neoadjuvant treatment (complete response)
Who can enter
- you have invasive breast cancer
- your cancer has one of the following tumour (T) stages - T1c, T2, T3, T4a, T4b, T4c or T4d
- your cancer has large amounts of HER2 protein (HER 2 positive) and does not have receptors to the oestrogen hormone (ER negative)
- doctors think that you are well enough to have neoadjuvant treatment and surgery
- you have had a tissue sample of your cancer taken (biopsy)
- your heart, liver and kidneys are working well
- you have satisfactory blood tests results
- you are well enough to carry out your normal activities, apart from heavy physical work (performance status of 0 or 1)
- you are willing to use reliable contraception during treatment and for at least 6 months afterwards if there is any possibility that you could become pregnant
- you are at least 18 years old
- you have had breast cancer on the same breast side or have any other cancer that needs treatment
- your cancer has spread to other parts of your body (metastatic)
- you have had chemotherapy
- you have had radiotherapy to a large area of your bone marrow
- you can’t have a combination of chemotherapy and targeted drugs, and breast cancer surgery for any reason
- have heart problems such as hypertension that isn’t controlled, your heart is unable to pump blood around the body properly, or you have narrowing of the heart blood vessels due to a build up of fat
- have a problem with the way your blood clots which causes a high risk of bleeding
- have any other serious medical condition or mental health problem that the trial team think could affect you taking part
- are pregnant or breastfeeding
- have had a live vaccine in the past 4 weeks
Trial design
- FEC with pertuzumab and trastuzumab. You then have docetaxel with pertuzumab and trastuzumab
- FEC and then docetaxel (FEC-T) with pertuzumab and trastuzumab
- docetaxel and carboplatin, and then pertuzumab and trastuzumab
You may also have samples of tissue taken from the lymph glands under your arm. This is common and part of your standard treatment.
- the whole of the breast (mastectomy)
- just the breast cancer (lumpectomy or breast conserving surgery)
- the lymph nodes under the arm (axillary clearance)
- after the first neoadjuvant treatment cycle
- after surgery
Hospital visits
- a physical examination
- blood tests
- mammogram and a breast ultrasound
- heart ultrasound (echocardiogram or MUGA)
- CT scan
- bone scan
Side effects
- pain at the site of the biopsy
- bruising and swelling of the breast
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Daniel Rea
Supported by
Cancer Research UK
University of Birmingham
Roche
Other information
This is Cancer Research UK trial number: CRUKE/16/025.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040