A trial looking at the follow up care after treatment for cancer of the cervix, womb, ovary and vulva (TOPCAT-G)
Cancer type:
Status:
Phase:
This trial compared two different types of follow up care for people who’d had treatment for a gynaecological cancer. This includes:
- cervical cancer
- womb (endometrial) cancer
- ovarian cancer
- vulval cancer
More about this trial
When this trial was done, people with gynaecological cancers often had hospital appointments for a number of years after they’d finished treatment. This is so the doctors could find out about any side effects they were having, and check for possible signs of the cancer coming back.
Researchers hope that a nurse led approach, which includes telephone support, could be helpful for people after treatment instead.
Half of the people in this trial had doctor led hospital appointments as usual. And half had nurse led follow up care.
The aims of this trial were to find out:
- if it is possible to recruit people to this type of study
- what people think of these different types of follow up
- whether it is possible to collect reliable information about the people taking part
Summary of results
The research team concluded that patients found nurse led follow up acceptable. And that it should be possible to run a larger trial looking at it in more detail.
This trial was open for people to join between 2015 and 2016, and the team published the results in 2018.
About this trial
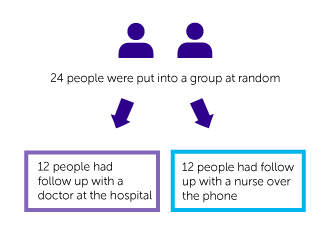
The people taking part were put into 1 of 2 groups at random:
- Group 1 had follow up as usual with their doctor at the hospital
- Group 2 had follow up with specialist nurses over the phone
Everyone saw a doctor about 3 months after they finished treatment. This was when they could decide whether take part in the trial or not. They then either:
- saw a doctor at the hospital at about 6 months and 9 months
- spoke to a nurse on the phone at about 4 months and 9 months
They also filled out some quality of life questionnaires before they started the follow up programme and just before each appointment or phone call.
When they reached a year after treatment, they continued to have the usual follow up for their type of cancer.
The research team looked at how happy people were with their follow up, how it had affected their quality of life, and how much it cost.
Results
The research team explained the trial to 44 people, and asked them if they’d like to take part.
Some people didn’t want to take part because they preferred to see a doctor, and didn’t want to be put into a follow up group at random. But more than half (55%) said they would like to take part. The team felt that overall this meant people found the idea of nurse led follow up appointments acceptable.
24 people were put into 1 of 2 groups at random:
- 12 people had usual follow up with a doctor
- 12 people had nurse led follow up
- 17 people had womb (endometrial or uterine) cancer
- 5 people had ovarian cancer
- 2 people had cervical cancer
They had already had an operation to remove the cancer. And about half had also had either radiotherapy or chemotherapy.No one had treatment for vulval cancer at the hospitals taking part during the time the trial was open for people to join (the recruitment period).
Quality of life
The research team analysed the quality of life questionnaires and found that there wasn’t much difference between the two groups.The people who had nurse led follow up had a slightly larger increase in quality of life. But the difference wasn’t big enough to say for sure that it was because of the type of follow up.
Cost of follow up
The research team also looked at how much the different types of follow up cost.They found that overall the cost of nurse led follow up was slightly less. But again it wasn’t a big enough difference to be sure that it was because of the type of follow up.
Conclusion
The research team concluded that nurse led follow up was acceptable to patients. And that it may cost less and improve quality of life. They feel it would be possible to run a larger trial to look at nurse led follow up in more detail.Where this information comes from
We have based this summary on information from the research team. The information they sent us has been reviewed by independent specialists (peer reviewed) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Mr Simon Leeson
Supported by
Betsi Cadwaladr University Health Board
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040