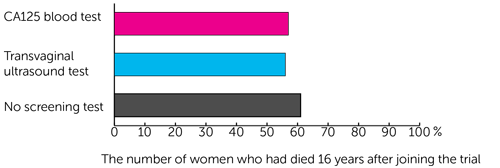A trial looking at screening the general population for ovarian cancer (UKCTOCS)
Cancer type:
Status:
Phase:
This trial looked at whether screening tests would be useful for diagnosing ovarian cancer. It was for women whose periods had stopped (they were post menopausal).
The trial was supported by Cancer Research UK. It was open for people to join between 2001 and 2005. The research team published long term results in 2021.
More about this trial
Cancer screening means looking for signs of cancer in people who don’t have any symptoms. Screening tests can help find early stage cancers. But the tests have to be reliable and accurate.
When this trial was done, there was no screening programme for ovarian cancer in the general population. In this trial, researchers tested the CA125 blood test and the transvaginal ultrasound scan. They wanted to find out if either were good enough to use as a screening test.
This trial was for women who:
- were between 50 and 74 years old
- had been through the
menopause 
- hadn’t been diagnosed with ovarian cancer
- didn’t have a family history or an inherited genetic change that increased their risk of ovarian cancer
The main aim of the trial was to see if either of these tests can help doctors diagnose ovarian cancer in people who don’t have symptoms.
Summary of results
The research team don’t recommend that either the CA125 blood test or a transvaginal ultrasound scan are used as screening tests for ovarian cancer.
Trial design
The women who joined this trial were put into a group at random. They had either a CA125 blood test, a transvaginal ultrasound scan or no screening test.
Women whose CA125 blood test showed a high chance of ovarian cancer then had a transvaginal ultrasound scan as well.
The research team compared the results for each group to find out:
- how many women were diagnosed with ovarian cancer
- how big the cancer was and whether it had spread (the stage)
- if screening helped people live longer
Results
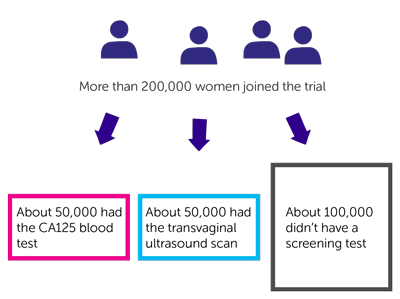
More than 200,000 women joined this trial. They were put into 1 of 3 groups at random:
- about 50,000 had the CA125 blood test
- about 50,000 had the transvaginal ultrasound scan
- about 100,000 didn’t have a screening test (the
control group  )
)
The women in the blood test group and the ultrasound group had screening tests every year from when they joined until 2011. On average this was 8 years.
The research team looked at the long term results in 2020. This was an average of 16 years after people had joined the trial.
They found that 2,055 women had been diagnosed with ovarian cancer. It was about 1 in 100 women (1%) in all 3 groups:
- 522 of those who had the CA125 blood test
- 517 of those who had the transvaginal ultrasound test
- 1,016 of those who didn’t have a screening test
The number of people diagnosed with early stage cancer was higher in the CA125 group compared the group who didn’t have screening. The research team looked to see if this affected how well treatment worked and how long women lived.
They looked at how many women in each group had died from their ovarian cancer. They found that this was similar in all 3 groups:
- 296 out of 522 women (57%) who had the CA125 blood test
- 291 of 517 women (56%) who had the transvaginal ultrasound test
- 619 of 1,016 women (61%) who didn’t have a screening test
Conclusion
The trial team concluded that using the CA125 blood test or transvaginal ultrasound scan to test for ovarian cancer didn’t help people live longer.
They do not recommend that either of these tests are used as screening tests for ovarian cancer in the general population. But even when a trial shows a test isn’t useful as a screening test, it adds to our knowledge and understanding of cancer and how to diagnose it.
This trial also shows how important it is to decide what to measure in a trial, and when. Some people in the CA125 group were diagnosed with cancer at an earlier stage. So it looked like CA125 may be a useful screening test. But the long term results showed that this didn’t mean people in that group lived longer.
Where this information comes from
We have based this summary on information from the team who ran the trial. The information they sent us has been reviewed by independent specialists ( ) and published in a medical journal. The figures we quote above were provided by the trial team. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Ian Jacobs
Supported by
Cancer Research UK
Department of Health
Medical Research Council (MRC)
NIHR Clinical Research Network: Cancer
Special Trustees of Barts and the London NHS Trust
Special Trustees of University College London Hospital
The Eve Appeal
Other information
This is Cancer Research UK trial number CRUK/03/029.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040