A trial looking at pembrolizumab before and after surgery for triple negative breast cancer (KEYNOTE 522)
Cancer type:
Status:
Phase:
This trial looked at pembrolizumab for early stage breast cancer that doesn’t have receptors for progesterone, oestrogen or HER2. This is called triple negative breast cancer.
The trial was open for people to join from 2017 to 2018. The research team presented the first results at a conference in 2019.
More about this trial
Some breast cancers have receptors for one or more of these:
- the hormone oestrogen
- the hormone progesterone
- the protein HER2
Doctors often treat these cancers with hormone therapies or a targeted treatment such as trastuzumab (Herceptin).
But some breast cancers don’t have any of these receptors. This type of cancer is called triple negative breast cancer. Doctors can’t use hormone therapies or trastuzumab to treat these cancers. They often use chemotherapy instead.
Researchers wanted to find out how well another type of targeted treatment called pembrolizumab would work.
Pembrolizumab (Keytruda) is a type of immunotherapy called a monoclonal antibody. It seeks out cancer cells by looking for a particular protein and attaching to it.
Doctors can give chemotherapy and other treatments before or after surgery, or both. People taking part in this trial had both.
There were 2 groups in this trial and people had either:
- standard chemotherapy and pembrolizumab before surgery, and more pembrolizumab after surgery
- standard chemotherapy and a dummy drug (placebo) before surgery, and more placebo after surgery
The main aims of the trial were to find out:
- if pembrolizumab and chemotherapy is better than chemotherapy alone for triple negative breast cancer
- more about the side effects of having pembrolizumab and chemotherapy at the same time
Summary of results
The results so far show that pembrolizumab and chemotherapy is better than chemotherapy alone, for triple negative breast cancer.
Trial design
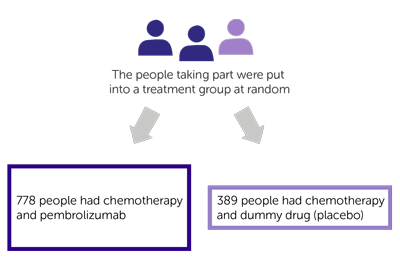
This trial was for people with early stage triple negative breast cancer that had not spread to anywhere else in the body. More than 1,100 people took part. They hadn’t had any treatment before joining the trial.
They were put into 1 of 2 treatment groups at random:
- 778 people had chemotherapy and pembrolizumab before surgery, and then pembrolizumab after surgery
- 389 people had chemotherapy and a dummy drug (placebo) before surgery, and then placebo after surgery
Results
The research team did the first analysis of the results in 2019. At that time, 602 people had been in the trial long enough for them to look at how well treatment before surgery had worked.
They looked at the number of people whose cancer had gone away completely when they had their operation. Doctors call this pathological complete response, or pCR.
They found the cancer had gone away in:
- 260 out of 401 people (65%) who’d had chemotherapy and pembrolizumab
- 103 out of 201 people (51%) who’d had chemotherapy and placebo
They also looked at the total number of people whose cancer had grown or spread, who had developed another cancer or had died. They looked at this 15 months after people had joined the trial.
They found it was:
- less than 1 out of 10 people (7%) who’d had chemotherapy and pembrolizumab
- just over 1 out of 10 people (12%) who’d had had chemotherapy and placebo
There is a difference between these groups, but it’s not big enough for the researchers to say for sure that it is because of the different treatments. It may be due to chance.
The research team looked in more detail at whether having a protein called PD-L1 on cancer cells affected how well treatment worked. Cancer cells that have the protein are called PD-L1 positive. And those that don’t have the protein are called PD-L1 negative.
They found that people whose cancer cells had the protein did better than those whose cancer cells didn’t have the protein. But the cancer went away in more people who had pembrolizumab in both groups.
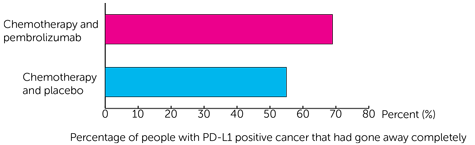
For people with PD-L1 positive cancer cells, the cancer had gone away completely in:
- 230 out of 334 people (69%) who had pembrolizumab and chemotherapy
- 90 out of 164 people (55%) who had placebo and chemotherapy
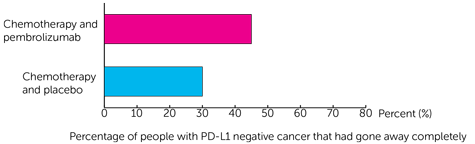
For people with PD-L1 negative cancer cells, the cancer had gone away completely in:
- 29 out of 64 people (45%) who had pembrolizumab and chemotherapy
- 10 out of 33 people (30%) who had placebo and chemotherapy
Side effects
Nearly everyone taking part had at least 1 side effect from treatment. Some were mild or short lived. About 3 out of 4 people in both groups had more severe side effects.
The side effects people had were mostly the usual side effects you’d expect from chemotherapy. The most common side effects were:
- feeling sick
- hair loss
- a drop in red blood cells and white blood cells
- extreme tiredness (fatigue)
Conclusion
The research team concluded that pembrolizumab and chemotherapy work better than chemotherapy alone, for early stage triple negative breast cancer. They suggest this combination should be used to treat this group of patients.
The team plan to do more analysis at a later stage to find out more about how well pembrolizumab works. We hope to update this page once these results are available.
Where this information comes from
We have based this summary on information from the research team. The information they sent us has been reviewed by independent specialists ( ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Peter Schmid
Supported by
Merck,Sharp & Dohme
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040