A trial looking at GSK3052230 alongside chemotherapy for lung cancer and mesothelioma of the chest
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is looking at a new drug called GSK3052230 alongside chemotherapy to treat lung cancer and mesothelioma. The trial is open to people who have a type of lung cancer called non small cell lung cancer and people who have mesothelioma in the chest (pleural mesothelioma).
More about this trial
Doctors can treat lung cancer and mesothelioma in the chest with  or
or  . If this doesn’t work, or your cancer has spread to another part of your body they can use
. If this doesn’t work, or your cancer has spread to another part of your body they can use  .
.
We know from research that some non small cell lung cancers and mesotheliomas have a change to a  called FGFR 1. This change in the FGFR 1 gene tells cancer cells to divide and grow.
called FGFR 1. This change in the FGFR 1 gene tells cancer cells to divide and grow.
GSK3052230 is a biological therapy. It stops the signals from the FGFR 1 gene that cancer cells use to divide and grow.
So before you join the trial the researchers need to test a sample of your cancer to see if you have the change in the FGFR 1 gene. The people taking part will have GSK3052230 with chemotherapy.
The aims of this trial are to find out
- The best dose of GSK3052230 to give with chemotherapy
- How safe it is to give GSK3052230 with chemotherapy
- How well GSK3052230 with chemotherapy works for non small cell lung cancer and mesothelioma in the chest
- What the side effects of GSK3052230 alongside chemotherapy are
- What happens to GSK3052230 in the body
- How it affects
quality of life 
Who can enter
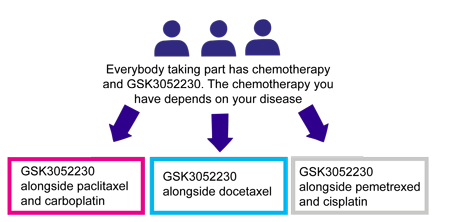
There are 3 treatment groups in the trial.
You may be able to join treatment groups 1 or 2 if you have a type of non small cell lung cancer (NSCLC) called a  that has spread to another part of your body.
that has spread to another part of your body.
For treatment group 1, you must not have had any treatment for your cancer. Or you had treatment with the aim to cure it more than 6 months ago.
For treatment group 2, you must have had a  that shows your cancer has got worse while having chemotherapy that included a
that shows your cancer has got worse while having chemotherapy that included a  . Your doctor can tell you this.
. Your doctor can tell you this.
You may be able to join treatment group 3 if you have mesothelioma in the chest that cannot be removed by surgery or has come back after having surgery or radiotherapy and you have not had chemotherapy.
As well as the above, you must also
- Have an area of cancer that can be measured on a scan
- Be well enough to carry out all your normal activities, apart from heavy physical work (performance status of 0 or 1) if you are joining treatment groups 1 and 3, and be well enough to be up and about for at least half the day (performance status 0, 1 or 2) for treatment group 2
- Have satisfactory blood test results
- Be willing to use reliable contraception for 2 weeks before starting treatment, during treatment and for 6 months after your chemotherapy or a month after stopping GSK3052230 (whichever is the latest) if there is any chance you or your partner could become pregnant
- Be at least 18 years old
You cannot join this trial if any of these apply. You
- Have cancer spread to your brain or you have an area of cancer pressing against your spine (spinal cord compression). You may be able to join if 4 weeks after having radiotherapy or surgery you have had a scan that shows the cancer spread to your brain hasn’t got any worse, you have no symptoms, and you aren’t taking steroids, or have been on the same dose of steroids for at least 4 weeks
- Have had a drug that works in a similar way to GSK3052230 (your doctor can tell you this). You can’t join treatment group 2 if you have had any other anti cancer treatment in the last month
- Have had another
biological therapy  in the last 6 weeks (your doctor can tell you this)
in the last 6 weeks (your doctor can tell you this) - Still have any moderate to serious side effects from any previous treatment apart from hair loss
- Have had another cancer apart from successfully treated non melanoma skin cancer or in situ carcinoma of the cervix
- Have any serious heart problems (the trial team can advise you about this)
- Have coughed up more than ½ teaspoon of red blood in the last 2 weeks
- Have an infection
- Have had major surgery or an injury in the past 4 weeks
- Have an ulcer, a wound or a bone fracture that hasn’t healed
- Have certain liver diseases (the trial team can advise you about this)
- Have any other serious medical condition or mental health problem that the trial team think could affect you taking part
- Are pregnant or breastfeeding
Trial design
This is an international phase 1/2 trial. The researchers need about 120 people to join.
There are 3 treatment groups in this trial. Everyone will have chemotherapy and GSK3052230. Your doctor will decide which chemotherapy you have depending on the disease you have and whether or not you have had treatment in the past.
The 3 treatment groups are
- People in group 1 have GSK3052230 alongside paclitaxel and carboplatin
- People in group 2 have GSK3052230 alongside docetaxel
- People in group 3 have GSK3052230 alongside pemetrexed and cisplatin
The first few people taking part will have a low dose of GSK3052230 alongside chemotherapy. If they don’t have any serious side effects, the next few people will have a higher dose of GSK3052230. And so on, until they find the best dose of GSK3052230 to give. This is called a dose escalation study.
You have GSK3052230 as a drip into a vein once a week. It takes 30 minutes each time. You can continue having GSK3055230 as long as it is helping and the side effects aren’t too bad.
You have chemotherapy as a drip into a vein once every 3 weeks. Each 3 week period is called a cycle of treatment. You have between 4 and 6 cycles of paclitaxel. You can continue to have docetaxel or pemetrexed and cisplatin as long as your cancer doesn’t get any worse or until your doctor thinks you have had the maximum benefit from it.
If you agree to take part in this study, the researchers will ask for a sample of your cancer from a previous  . If a sample from a previous biopsy isn’t available you must agree to have a biopsy to take part in the trial. The team will use the sample to test for the change in the FGFR 1
. If a sample from a previous biopsy isn’t available you must agree to have a biopsy to take part in the trial. The team will use the sample to test for the change in the FGFR 1  .
.
They will also use the tissue sample to look for substances called  that may help them measure how well GSK3052230 is working. You don’t have to agree to this if you don’t want to, you can still take part in the trial.
that may help them measure how well GSK3052230 is working. You don’t have to agree to this if you don’t want to, you can still take part in the trial.
During the trial the team will ask for some extra blood samples. They will use these to
- Find out about what happens to GSK3052230 in the body
- Look for
antibodies  that your body’s immune system makes that could affect how well GSK3052230 works
that your body’s immune system makes that could affect how well GSK3052230 works - Look for changes in biomarkers that could measure how well GSK3052230 is working
The researchers also want to look at the genes in your blood cells. The differences in people’s genes may help explain more about how GSK3052230 works. You don’t have to agree to this, you can still take part in the trial.
The trial team will ask you to fill out a questionnaire before you start treatment, every 3 weeks during treatment, when you finish treatment and a month later. The questionnaire will ask about side effects and how you’ve been feeling. This is called a quality of life study.
Hospital visits
You see the doctor to have some tests before taking part in the trial. These tests include
- A physical examination
- Blood tests
- Heart trace (
ECG  )
) - Heart scan (
ECHO  )
) - CT scan, MRI scan or PET CT scan
- Bone scan
- Urine test
Breathing tests  (people having pemetrexed and cisplatin only)
(people having pemetrexed and cisplatin only)- Eye test
During treatment you see the doctor every 3 weeks for a physical examination and blood tests. You have a CT scan, MRI scan or PET CT scan every 6 weeks for a year and then every 12 weeks.
If you are in treatment arm 3, you have breathing tests again every 6 weeks throughout treatment.
At the end of treatment you see the doctor and have the same tests you had at the beginning of the trial. You then see the doctor a month later for a physical examination, blood tests and a scan.
Side effects
GSK3052230 is a new drug and there may be side effects we don’t know about yet. The most common side effects so far include
- Diarrhoea or constipation
- Tiredness (fatigue)
- Feeling or being sick
- Headache
- Pain where your cancer is
- Swelling of the feet or legs
- Shortness of breath
- Tummy (abdominal) pain
- Back and joint pain
- Cough
- Skin rash
- Loss of appetite
You may have an allergic reaction when you are having GSK3052230 or for up to several hours afterwards. This can cause
- Swelling of the face, mouth, throat, lips or tongue
- Difficulty breathing
- Skin rash and itchiness
- Dizziness and fainting
- Low blood pressure
- Headache
- Feeling sick
You may have eye problems such as sensitivity to light, changes to your vision, dry eyes or eye pain.
You may have weakness in your legs caused by muscle weakness or nerve damage.
If you have any signs of an allergic reaction, eye problems or leg weakness, you must contact your doctor right away.
We have more information about the side effects of
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Vanessa Potter
Supported by
Experimental Cancer Medicine Centre (ECMC)
GlaxoSmithKline (GSK)
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040