A study looking at seprehvir to treat mesothelioma
Cancer type:
Status:
Phase:
This study looked at seprehvir for people with mesothelioma of the lungs (pleural mesothelioma). Seprehvir is also called HSV1716.
It was open for people to join between 2012 and 2016. The team published the results in 2020.
More about this trial
Pleural mesothelioma is cancer that starts in the lining around the lungs. When this study was done, doctors often used surgery, radiotherapy or chemotherapy to treat mesothelioma.
Researchers wanted to find out whether seprehvir could be a useful treatment for mesothelioma that couldn’t be removed with surgery.
Seprehvir is also called HSV1716. It is a modified version of the herpes simplex virus (HSV). It can’t cause a herpes infection because it’s inactive. But it can get into cancer cells, and help stimulate the immune system to kill them.
Researchers already knew that seprehvir can get into cancer cells and help to kill them. And other studies had shown that it is safe and may help people with other types of cancer.
The aim of this study was to find out:
- what the side effects are for people with mesothelioma
- what happens to the virus in the mesothelioma cells
- whether seprehvir may be able to help stop mesothelioma growing
Summary of results
The research team found that seprehvir didn’t cause too many side effects. And that it did cause changes in mesothelioma cells and the immune system.
Study design
This study was for people with mesothelioma that couldn’t be removed with surgery. Some people taking part had had chemotherapy already, and some had not.
Seprehvir is a liquid. In this study, people had treatment through a small tube which went into the space around the lungs. This is called intrapleural treatment. The research team hoped this would cause fewer side effects than having treatment into a vein.
The first few people had 1 dose of seprehvir. The next few people had 2 doses, one week apart. And the next few people had 4 doses, all one week apart. This is called dose escalation. Researchers do this so they can check carefully for side effects before giving people more treatment.
Results
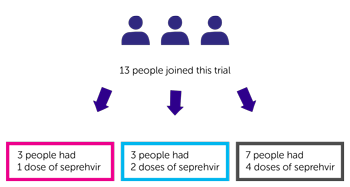
A total of 13 people joined this trial:
- 3 people had 1 dose of seprehvir
- 3 people had 2 doses of seprehvir
- 7 people had 4 doses of seprehvir
Everyone taking part had a small tube that went through the skin and into the space around their lungs. This tube is called a pleural catheter.
Researchers used the pleural catheter to:
- give treatment to the area around the lungs
- drain off any excess fluid that had built up
- take samples of fluid and cells from the space around the lungs
The research team looked at levels of different proteins in the fluid samples they took from 12 people who took part.
They found the genetic code (DNA) of the virus in the fluid around the lungs of 9 out of the 12 people. This means the virus was able to get into the cancer cells that were in the fluid.
They also looked at whether seprehvir had an effect on people’s immune system.
They found there was an increase in a protein called IgG in 9 people. IgG is an  produced by the immune system when we get an infection. An increase in IgG means the treatment helped the immune system to recognise (and kill) cancer cells.
produced by the immune system when we get an infection. An increase in IgG means the treatment helped the immune system to recognise (and kill) cancer cells.
Cytokines are also proteins that are part of our immune system response. The researchers looked at the level of cytokines in the pleural fluid of 11 people. They found it had increased in 8 of them.
Side effects
Some people taking part had side effects such as:
- flu like symptoms
- a temperature
- extreme tiredness (fatigue)
These side effects mostly affected people who had 4 doses of seprehvir.
No one taking part had side effects serious enough to suggest they were having too many doses of seprehvir.
One person decided to stop taking part in the trial because of problems with their pleural catheter.
How well it worked
It’s hard to say how well seprehvir worked as a treatment for mesothelioma because of the small number of people in the trial.
The team looked at whether the cancer got any better and found that it:
- didn’t get smaller in anyone
- stayed the same in 6 people
- continued to grow in 6 people
Conclusion
The research team concluded that seprehvir didn’t cause too many side effects. And that it may be a useful treatment for people with mesothelioma that can’t be removed with surgery.
They suggest it is looked at in other trials in combination with other targeted cancer treatments.
Where this information comes from
We have based this summary on information from the research team. The information they sent us has been reviewed by independent specialists ( ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Prof Penella Woll
Supported by
Experimental Cancer Medicine Centre (ECMC)
Sheffield Teaching Hospitals NHS Foundation Trust
VIRTTU Biologics
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040